Acidic oxygen evolution reaction via lattice oxygen oxidation mechanism: progress and challenges
Abstract
The lattice oxygen mechanism (LOM) plays a critical role in the acidic oxygen evolution reaction (OER) as it provides a more efficient catalytic pathway compared to the conventional adsorption evolution mechanism (AEM). LOM effectively lowers the energy threshold of the reaction and accelerates the reaction rate by exciting the oxygen atoms in the catalyst lattice to directly participate in the OER process. In recent years, with the increase of in-depth understanding of LOM, researchers have developed a variety of iridium (Ir) and ruthenium (Ru)-based catalysts, as well as non-precious metal oxide catalysts, and optimized their performance in acidic OER through different strategies. However, LOM still faces many challenges in practical applications, including the long-term stability of the catalysts, the precise modulation of the active sites, and the application efficiency in real electrolysis systems. Here, we review the application of LOM in acidic OER, analyze its difference with the traditional AEM mechanism and the new oxide pathway mechanism (OPM) mechanism, discuss the experimental and theoretical validation methods of the LOM pathway, and prospect the future development of LOM in electrocatalyst design and energy conversion, aiming to provide fresh perspectives and strategies for solving the current challenges.
Keywords
INTRODUCTION
With the increasing demand for renewable energy sources, energy conversion and storage technologies have become a hot area of scientific research in today's world[1-5]. Oxygen evolution reaction (OER) plays a crucial role as a key link in these technologies, especially in systems such as water decomposition and metal-air batteries. OER involves the extraction of oxygen from water molecules, a process that not only requires a large amount of energy input, but is also a rate-determining step that constrains the efficiency of the overall system[6-9].
Proton Exchange Membrane (PEM) technology shows great potential for applications in areas such as fuel cells and water splitting due to its high efficiency, fast response and good stability[10,11]. However, the acidic environment in the PEM places higher demands on the stability and activity of the OER catalyst, which is located at the anode and is required to oxidize H2O into oxygen (O2) and protons (H+)[12,13]. The energy efficiency of this process directly affects the performance of the entire water decomposition system[14]. Meanwhile, OER in metal-air batteries determines the charging efficiency and cycle stability of the battery[15]. Therefore, the development of efficient OER catalysts to lower the energy barrier of the reaction and increase the energy conversion efficiency is key to the commercialization of these technologies.
It is well known that OER in acidic media faces a series of special characteristics and challenges. Firstly, the high proton concentration under acidic conditions is favorable for H+ generation and migration, but it also puts higher demands on the stability of electrocatalysts. Many electrocatalysts are easily corroded or dissolved in an acidic environment, which leads to a rapid decrease in catalytic performance[16]; secondly, the high proton activity in an acidic medium may cause side reactions with the catalyst surface, which affects the efficiency of OER[17]. In addition, the dissociation of H2O is low under acidic conditions, which may limit the formation and conversion of key intermediates in the OER process[18].
Currently, most research on OER electrocatalysts is indeed conducted in alkaline media, mainly due to the fact that OER processes in alkaline media typically have more favorable reaction kinetics and lower energy barriers. However, OER studies in acidic media are equally important because of their unique advantages in applications such as proton exchange membrane water electrolysis (PEMWE). The differences between acidic and alkaline media in various aspects are analyzed in detail below, and how these differences affect the properties of lattice oxygen mechanism (LOM) in acidic media: (i) Influence of the medium on the OER reaction path. In alkaline media, the OER reaction path is relatively straightforward, mainly involving steps such as OH- adsorption, deprotonation, and O-O bond formation, and OER kinetics in alkaline media are more favorable, with faster reaction rates. The OER reaction path in acidic media is more complex and requires high energy to break the strong covalent OH bond of H2O, so the kinetics are slower. In addition, OER in acidic media may involve the LOM mechanism; lattice oxygen in the catalyst is involved in the reaction, which increases the complexity and challenge of the reaction. The higher kinetic energy barrier for OER in acidic media requires more efficient catalysts to lower the energy barrier and achieve efficient OER; (ii) Effect of medium on catalyst stability. Most metal catalysts show better stability in alkaline media because alkaline solutions are less corrosive to metals; (iii) Acidic media have a strong corrosive effect on metal catalysts, which poses a serious challenge to the long-term stability of electrocatalysts. In acidic media, the LOM pathway may accelerate the degradation of the catalyst, as the involvement of lattice oxygen may lead to the destruction of the catalyst structure and loss of active sites.
For this reason, there are differences in electrocatalyst design, which, in alkaline media, is mainly concerned with the optimization of active sites and the regulation of the reconstruction process. For example, by adjusting the composition, structure and morphology of the catalyst, its OER performance can be optimized. In acidic media, on the other hand, electrocatalyst design requires more consideration of its corrosion resistance and stability. This includes the selection of acid-resistant catalyst materials, the optimization of electrocatalyst dispersion and electronic structure, and the design of electrocatalyst structures that inhibit unfavorable reaction pathways. For example, the introduction of inexpensive metal elements into the electrocatalysts can enhance the stability and OER performance of the electrocatalysts in acidic media by modulating their electronic structure and local coordination environment. In terms of application prospects, OER electrocatalysts in alkaline media have a wide range of prospects for application in fields such as alkaline water electrolysis. However, the commercial application of alkaline water electrolysis systems is somewhat limited due to their drawbacks such as low current density and uncompact design. OER electrocatalysts in acidic media have unique advantages in applications such as PEM water electrolysis. PEMWE systems are more suitable for large-scale commercial applications due to their advantages such as compact design, higher voltage efficiency and higher gas purity. Therefore, the development of efficient and stable acidic OER catalysts is of great significance in promoting the advancement of PEM water electrolysis technology.
Understanding the reaction mechanism in the study of electrocatalytic OER is essential for designing efficient electrocatalysts[19]. The catalytic mechanisms of OER mainly include adsorption evolution mechanism (AEM), oxide pathway mechanism (OPM) and LOM, as shown in Figure 1A-C[20-22]. These mechanisms define the process of O2 molecule formation on the surface of different catalytic materials and how they affect the kinetics and efficiency of OER. Researchers have been exploring novel catalysts and catalytic mechanisms. Among them, the LOM has received much attention due to its unique reaction pathway and potential advantages in acidic media [Figure 1D]. LOM is expected to lower the energy barrier of the reaction and improve the stability and activity of the catalyst by utilizing the oxygen atoms in the catalyst lattice to participate in the OER process. In this paper, we will discuss in detail the discrimination of LOM, its application in acidic OER, its differences with conventional AEM and OPM, and the potential and challenges of LOM in electrocatalyst design and energy conversion technology. With these discussions, we expect to provide novel perspectives and strategies for the design of more efficient and stable electrocatalysts for OER in acidic environments.
Figure 1. The OER catalytic cycle based on AEM (A); LOM (B) and OPM (C); (D) Developmental process and crucial stages in the emergence of lattice oxygen oxidation mechanisms in OER electrocatalysts. OER: Oxygen generation reaction; AEM: Adsorption evolution mechanism; LOM: Lattice oxygen mechanism; OPM: Oxide pathway mechanism.
GENERALIZED MECHANISM OF ELECTROCATALYTIC OER
Understanding the mechanism of OER in acids has profound scientific significance and practical application value for the development of energy conversion and storage technologies[23-25]. An in-depth understanding of the OER mechanism can help to reveal the intrinsic laws of the reaction kinetics, which can provide a theoretical basis for the design and optimization of high-efficiency electrocatalysts[26,27]. By identifying the rate-determining steps in the reaction process, the performance of the catalyst can be targeted to improve the energy conversion efficiency by reducing the required overpotential[28]. An understanding of the acidic OER mechanism can help to improve catalyst stability. Under acidic conditions, electrocatalysts are typically exposed to harsher chemical corrosion environments, and understanding the various aspects of the OER process can guide researchers in developing more durable materials that will extend the life of the electrocatalysts.
The understanding of the acidic OER mechanism is also relevant to the realization of cost-effectiveness[29,30]. By revealing the active site requirements for different catalytic mechanisms, the utilization of non-precious metals or low-cost materials can be facilitated and the production cost of catalysts can be reduced, making clean energy technologies more economical and competitive in the market[31]. Understanding the mechanism of acidic OER is also crucial for system optimization. This involves not only the selection and design of electrocatalysts, but also the overall structure of the electrolyzer, the composition of the electrolyte, and the optimization of the operating conditions to achieve efficient operation of the whole system[32,33]. Finally, with the deepening of the understanding of the acidic OER mechanism, new ideas and methods can be provided for scientific research and technological development in related fields, which will promote the innovation of renewable energy technologies and contribute to the realization of sustainable development of society. Therefore, the study of the acidic OER mechanism is not only a cutting-edge issue in the research of chemistry and materials science, but also an important topic in the field of energy science and technology[34-36].
Firstly, the AEM [Figure 1A] was the first widely accepted OER mechanism based on the gradual adsorption and evolution of H2O on the surface of the electrocatalyst[37]. It involves a series of intermediates, such as *OH, O and OOH, which form oxygen through successive proton-electron pair transfer steps. In AEM, the reaction involves the successive formation and transformation of a series of intermediates in the adsorbed state, and the process can be represented as the following primitive reaction steps[38,39]:
The * here denotes the active site, which can be a metal/metal oxide/single atom or even a non-metallic compound. The AEM-driven OER catalytic cycle is mainly described as: Adsorption of H2O molecules (1): H2O molecules are adsorbed onto the active site of the catalyst to form hydroxide ions (*OH). The adsorbed *OH evolves further and is converted to an oxygen intermediate by electron acquisition and one-step deprotonation [*O, (2)]. *O is further oxidized by combining another *OH and a deprotonated electron to form preoxygenated intermediates [*OOH, (3)]. Finally, the second step of *OOH desorbs protons and electrons, forming and releasing oxygen (O2) back into the gas phase while regenerating the reactive site
The OPM [Figure 1C] is a less common mechanism usually associated with the oxide component of a multiphase catalyst[21,40]. In OPM, the oxide component of the catalyst is directly involved in the generation of O2 without the need to pass through intermediates adsorbed on the surface. OPM is an OER mechanism observed on some oxide catalysts[41]. Unlike AEM, OPM does not rely on intermediates adsorbed on the catalyst surface but involves the direct participation of oxide components. The reaction pathway is mainly summarized in the following steps[42]:
Where M-M-O denotes metal oxides, and the reaction starts with the activation of the oxide component in the electrocatalyst lattice, which may involve either the pre-activation of lattice oxygen or the direct involvement of lattice oxygen (5). Under electrocatalysis, the oxide components of the lattice are oxidized to form metal-oxygen (M-O) intermediates in a highly oxidized state (6). Oxygen-oxygen bonds are formed through interactions and rearrangements between lattice oxygens to produce O2 molecules (7). The generated oxygen molecules are released from the electrocatalyst surface to complete the OER process. After the release of oxygen, the catalyst lattice may need to be regenerated by adsorbing oxygen from the electrolyte to maintain the catalytic cycle (8)[43,44].
In OPM, O2 generation may not involve explicit surface adsorbed state intermediates, but rather through direct oxidation and recombination of lattice oxygen. This mechanism may be more common in catalysts with specific lattice structures and electronic properties, such as those with oxide or spinel structures[45,46]. The specific reaction steps of OPM may vary depending on the chemical composition and structure of the catalyst. The demonstration of OPM mechanisms usually relies on in situ spectroscopic techniques such as X-ray absorption spectroscopy (XAS), neutron scattering, or Mössbauer spectroscopy, which can provide direct information about the state and dynamics of lattice oxygen[47,48]. In addition, theoretical calculations help to understand the thermodynamic and kinetic properties of the OPM mechanism[49,50].
In early studies, OER was mainly understood through the AEM, which involves the gradual adsorption and evolution of H2O on the electrocatalyst surface. However, with the exploration of efficient electrocatalysts, especially solid-phase oxides and hydroxides, researchers found that the lattice oxygen of the catalyst itself would participate in the reaction at the OER potential, a phenomenon that eventually developed into the lattice LOM[51,52]. The proposed LOM mechanism breaks through the limitations of traditional AEM and provides a new explanation for solid-phase catalysts with high catalytic activity. The in-depth study and understanding of the LOM promotes a completely new perspective on the design of OER electrocatalysts, especially in the enhancement of electrocatalysts activity and stability[53]. With the development of in situ spectroscopic techniques and theoretical calculations, the LOM mechanism has been further verified and thoroughly explored[34,50].The lattice LOM refers to the direct participation of oxygen atoms in the catalyst lattice in the formation of O2. This mechanism is particularly important in certain metal oxide electrocatalysts with specific lattice structures. The reaction steps can be given in more detail as[54,55]:
Where M denotes the metal, the transfer of electrons in the step may be accompanied by the transfer of protons, depending on the acidity or alkalinity of the medium. The basic principle of the lattice LOM is the direct participation of oxygen atoms in the catalyst lattice in electrocatalytic reactions. Below are several key steps in the pathway of the LOM mechanism. Activation of lattice oxygen: O atoms in the catalyst lattice are first activated, usually through the formation of oxygen vacancies. These oxygen vacancies increase the activity of the lattice oxygen, making it more likely to participate in subsequent redox reactions [(9) - (11)]. Formation of oxygen vacancies [Vo, (12)]: the formation of Vo is a key step in the LOM. Oxygen vacancies can be generated by external doping, lattice defects or external electric fields, which provide the necessary conditions for lattice oxygen activation and participation in the reaction. Oxidation of lattice oxygen (13): The activated lattice oxygen atoms are further oxidized to form oxide species (*O or *OOH). These oxide species can react with water molecules or other intermediates adsorbed on the catalyst surface to further form oxygen. Regeneration of Lattice Oxygen (14): after the release of O2, the lattice oxygen sites need to be regenerated to maintain the catalytic cycle. This is usually achieved by adsorption of new O atoms from the electrolyte or by redistribution of electrons[56,57]. The thermodynamic possibilities of the LOM mechanism can be gauged by the energy band structure and mutual position of the metal center and oxygen ligand[58].
LOM may have the following advantages over AEM and OPM. Inherent Activity: LOM exhibits higher inherent activity than AEM and OPM by directly utilizing the oxygen atoms in the catalyst lattice to participate in the OER, potentially reducing the activation energy of the reaction. Acid resistance: LOM may have better stability in acidic media, as the adsorbed state intermediates involved in AEM may be more susceptible to proton attack, leading to catalyst surface reconfiguration or inactivation. Electronic structure modulation: LOM may optimize the redox capacity of lattice oxygen by modulating the electronic structure of the catalyst, achieved by n-type or p-type doping, which enhances the lattice oxygen activity. Energy efficiency: LOM may reduce the co-transfer step of protons and electrons and reduce the energy loss during OER, especially at high current densities. Long-term stability: LOM may reduce the remodeling of the catalyst surface during the OER process, thus improving its long-term stability, which is particularly important for OPM that may undergo structural changes during the reaction. Experimental validation: The activity and stability of LOM have been experimentally validated, including the use of in situ spectroscopic techniques combined with theoretical calculations, to gain insight into the catalytic process of LOM under acidic conditions.
Although the LOM provides a fresh interpretation of the activity of OER electrocatalysts, there are still challenges to be faced in this area of research, including the singularity of the means of catalyst material synthesis, the problem of lattice oxidation leading to the reconstruction of the catalyst surface, the need for further development of in-situ spectroscopic characterization techniques, and the construction of a more realistic catalyst model for theoretical simulations. Future research needs to make progress in these areas to fully exploit the potential of the LOM mechanism in OER catalyst design.
CHARACTERIZATION TECHNIQUES FOR IDENTIFYING LOM
LOM is a special type of OER mechanism in which oxygen atoms in the catalyst lattice are directly involved in the reaction, rather than proceeding only through species adsorbed on the surface. This mechanism may dominate in some electrocatalyst systems and lead to specific reaction kinetics. In situ electrochemical spectroscopy can assist in identifying this mechanism by capturing characteristic signals (specific spectral peaks or electrochemical reactions) associated with LOM, special molecular probes, isotopically labeled tracers and other methods[48,59-61].
Chemical methods used to identify LOM
In situ electrochemical-spectroscopic techniques
During the OER process, the electrode surface undergoes a series of complex chemical changes, including electron transfer, chemical bond breaking and formation. By capturing the spectral signals during these changes, in situ electrochemical spectroscopy can provide direct evidence about reaction intermediates, energy states, and electronic structures. These spectral signals can reveal the species involved in the reaction process, their transformation pathways, and the kinetic properties of the reaction[62-64]. For the resolution of OER mechanisms, in situ electrochemical spectroscopy is able to distinguish between different reaction paths and intermediate states. Oxygen atoms in the electrocatalysts lattice are directly involved in the reaction in the LOM. This mechanism typically involves the migration and transformation of oxygen atoms within the catalyst, resulting in specific spectral signal changes[65]. By capturing these changes, in situ electrochemical spectroscopy techniques can identify the presence of the LOM mechanism and further investigate its details[66-68]. In addition, in situ electrochemical spectroscopy techniques can be combined with other characterization tools, such as electrochemical impedance spectroscopy and surface analysis techniques, to provide more comprehensive information. The combined use of these techniques can further validate and complement the spectroscopic data to reveal the OER mechanism more accurately[69].
The application of in situ spectroscopy techniques to alkaline OER has been a remarkable achievement, providing important information for understanding reaction kinetics and electrocatalyst optimization[70]. However, the complexity of acidic OER requires more precise analytical tools. In this context, the development and application of in situ electrochemical spectroscopic testing techniques have enabled researchers to directly observe and differentiate between AEM, lattice LOM, and other possible reaction pathways under acidic conditions. Xin et al.[71]. have carried out an in-depth OER reaction mechanism study on the Fe-Co(OH)2/Fe2O3 heterostructure and characterized it by in situ Raman spectroscopy [Figure 2A]. The experimentally observed Raman peaks were located at 808, 914, 980 and 1063 cm-1, all associated with oxygenated intermediates, especially the peak at 1063 cm-1, which was considered to be the characteristic signal of oxide ions (*O2-). This *O2- peak was enhanced with increasing potential. In addition, using molecular probe techniques (NO2- and SCN-) and Density Functional Theory (DFT), the team confirmed the transition of the catalytic mechanism in the OER process, the transition from the AEM mechanism of Co(OH)2 to the LOM mechanism of Fe-Co(OH)2/Fe2O3. Yao et al.[72]. also performed in situ Raman spectroscopy in order to verify the LOM reaction mechanism of acidic OERs of Ru-UiO-67-bpydc and
Figure 2. (A) In situ Raman spectra of Fe-Co(OH)2/Fe2O3 (Copyright 2020, John Wiley and Sons[71]) and Ru-Uio-67-bpydc (B) (Copyright 2023, Elsevier[72]) and FTIR spectra 3D Co3O4/NC (C) (Copyright 2023, Elsevier[73]) at various potentials; (D, E) 16O18O-labeled CV of mass spectra and in situ Raman spectra after different times (F) (Copyright 2022, Springer Nature[74]). FTIR: Fourier transform infrared spectroscopy.
In situ electrochemical spectroscopy combines the advantages of electrochemistry and spectroscopy, allowing researchers to monitor chemical changes on the electrode surface in real time during the reaction process. This real-time monitoring capability allows us to directly observe the dynamic behavior of catalysts and reaction intermediates during the OER process, providing insight into the reaction mechanism.
pH-dependent and probe-recognized LOM mechanisms in solution electrochemistry
The LOM mechanism involves the direct involvement of lattice oxygen in the OER process, which is typically observed in oxide electrocatalysts[44]. The TetraMethylAmmonium (TMA) molecules can interact with lattice oxygen sites to specifically identify the LOM process by monitoring the oxidative or reductive behavior of the TMA, a probe designed specifically to interact with a target catalyst or reaction intermediate, thus providing direct evidence of the LOM process. When the TMA probe comes into contact with the catalyst, it may selectively react with or be attracted to the lattice oxygen, resulting in a measurable electrochemical signal[57,75,76]. On the other hand, the charge state and reactivity of the electrocatalyst surface may change in different pH environments[77,78], thus affecting the efficiency and reaction rate of the LOM process. The electrocatalyst surface may undergo protonation or deprotonation when the solution pH changes, and these changes can affect the activity, mobility and stability of the lattice oxygen. In electrochemical testing, the effect of pH on the LOM process can be inferred by measuring parameters such as current, potential, and reaction rate of the OER at different pH conditions[79,80]. A significant change in OER performance with increasing or decreasing pH may indicate that the LOM process is pH sensitive, as changes in pH may affect the release of lattice oxygen or the ability to participate in the reaction.
During LOM-driven electrocatalysis, the electrocatalyst surface undergoes sequential oxidation, exchange and release of lattice oxygen ligands involving negatively charged oxygenated species such as *O22-/*O2-. The TetraMethylAmmonium (TMA+) is a positively charged ion that interacts with lattice oxygen (as a negatively or partially negatively charged site), and by monitoring the electrochemical response of TMA upon contact with the catalyst, the activation state and degree of involvement of lattice oxygen can be indirectly observed to discern the mechanism. In electrochemical experiments, TMA+ acts as an electron probe that can track the oxygenated species during the reaction process, indirectly verifying the activation of the LOM mechanism in OER. The OER mechanisms of catalysts such as Fe-Co(OH)2/Fe2O3[71] [Figure 3A], Fe-CoP[81] [Figure 3B], and IrOx/Y2O3[82] [Figure 3C] under alkaline conditions, as well as of Ni-IrRu[36] and V-RuO2[83] under acidic conditions, have been confirmed by this probe approach. Similarly, the electrocatalysts exhibited clear pH dependence in electrolytes of different pH values, and their catalytic activity increased significantly with pH [Figure 3D-I]. This property suggests that the rate of OER under LOM may not be limited by the conventional ligand proton-electron transfer mechanism. In contrast, the low pH sensitivity of AEM-type electrocatalysts is consistent with their inherent proton-electron transfer step. In the AEM process, uncoordinated proton-electron transfer and the formation of negative oxygen intermediates are the key steps in the conversion of the active site to the final oxygen product[84,85]. These observations provide new perspectives for understanding the catalytic behavior of OER under different pH conditions and help to reveal the kinetic properties under diverse catalytic mechanisms.
Figure 3. OER performance in solutions with and without TMA: (A) Fe-Co(OH)2/Fe2O3 (Copyright 2020, John Wiley and Sons[71]); Plot of OER performance in electrolytes at different pH solution conditions and the corresponding logarithm of current density versus OER path: (B-C) Fe-Co(OH)2/Fe2O3 (Copyright 2020, John Wiley and Sons[71]); (D) B, Fe-CoP (Copyright 2024, John Wiley and Sons[81]); (E-F) B, Fe-CoP (Copyright 2024, John Wiley and Sons[81]); (G) IrOx/Y2O3 (Copyright 2023, John Wiley and Sons[82]); (H-I) IrOx/Y2O3 (Copyright 2023, John Wiley and Sons[82]). OER: Oxygen generation reaction; TMA: TetraMethylAmmonium.
Combining these two points, electrochemical tests were carried out in solutions of distinct pH values in combination with the use of TMA probes. This not only helps us to confirm the existence of the LOM mechanism, but also reveals how pH affects the LOM process, providing valuable information for optimizing catalyst design and improving OER performance. We can deeply understand and explain the LOM mechanism of OER in principle, providing important theoretical support and practical guidance for research in the field of energy conversion and storage.
Isotopic tracer discrimination
By using isotopically labeled H2O or O2, it is possible to trace the origin and destination of oxygen atoms in the OER process, thus verifying whether lattice oxygen is involved in oxygen production[86,87]. Combined with the practical principles of isotope labeling techniques, using 18O probes or H (D) isotope probes, we can accurately trace the transfer paths of oxygen and hydrogen atoms, thus verifying whether lattice oxygen participates in the OER process, and further discerning the LOM mechanism[88]. In OER studies, 18O probes and H (D) isotope probes play a key role; 18O probes are used to trace the origin and destination of oxygen atoms, while H isotope probes can reveal the reaction behavior of hydrogen atoms. When these isotope probes are involved in OER reactions, we can trace the transfer paths of atoms by analyzing the isotopic composition of the reaction products[89,90]. In the case that lattice oxygen is involved in the OER process, then in experiments using 18O probes, compounds containing 18O will be detected in the reaction products, which is direct evidence of the involvement of lattice oxygen. Similarly, hydrogen isotope probes can help us understand the behavior of hydrogen atoms in a reaction and determine whether a hydrogen oxidation or reduction process is involved[91]. By comparing the reaction behavior of isotope probes under various conditions (multiple electrocatalysts, distinct reaction conditions), we can gain a deeper understanding of the working principle of the LOM mechanism and verify whether it is consistent with experimental observations. Fan et al.[92]. carried out DEMS tests for 18O isotope labeling [Figure 4A]. 32O2 and 34O2 were generated by the OER, providing strong evidence for the involvement of lattice oxygen in the OER process, with O2 consisting of lattice oxygen from Ni-FeNi3/Ni0.5-bFe0.5-yMo1.5Ox and O atoms from the electrolyte [Figure 4B]. Yao et al.[72]. discovered that when RuO2 and Ru-UiO-67-bpydc were placed as acidic OER electrocatalysts in a solution of TMA+ [Figure 4C], the OER activity was significantly reduced due to the binding of *O22- intermediates to TMA+, which slowed down the release of O2. Further, the involvement of lattice oxygen in the OER process was directly confirmed by DEMS detection of periodic signals of 18O-labeled oxygen (18O16O) [Figure 4D]. Similarly, IrOx/Y2O3-EC [Figure 4E-F][82], MoNiFe (oxy)hydroxides
Figure 4. (A) Schematic representation of DEMS detection of the isotope O2; (B) Ni-FeNi3/Ni0.5-bFe0.5-yMo1.5Ox as the DEMS signal of the 18O isotope labeled product under electrocatalyst (Copyright 2024, John Wiley and Sons[81]); (C) OER behavior of Ru-UiO-67-bpydc in different solutions (with and without isotopic substitution and TMA+); (D) DEMS tests for 18O16O and 18O18O signals were performed on 18O-labeled Ru-UiO-67-bpydc catalysts in 0.5 M H2SO4 and H216O (Copyright 2023, Elsevier[72]); 18O isotopically labeled IrOx/Y2O3-EC (E) and IrOx/Y2O3-EC (F) for DEMS testing (Copyright 2023, John Wiley and Sons[82]); Mass spectral cyclic voltametric curves of 16O18O-labeled MoNiFe (oxy)hydroxide (G) and 18O2-labeled MoNiFe (oxy)hydroxide (H) (Copyright 2022, Springer Nature[74]); The O2 product of GB-RuO2 labelled on the 18O surface was analyzed by in situ Raman spectroscopy (I) and DEMS signal analysis (J) (Copyright 2024, John Wiley and Sons[93]); (K) 18O labeled RuIrCaOx and RuIrOx electrocatalysts producing 16O18O (m/z = 34) signals in O2 DEMS measurements (Copyright 2020, John Wiley and Sons [94]); (L) DEMS signals (36O2, 34O2 and 32O2) of Rh-RuO2/G electrocatalysts in aqueous H218O sulphuric acid solution (Copyright 2023, Springer Nature[95]). OER: Oxygen generation reaction; DEMS: Differential electrochemical mass spectrometry.
It should be pointed out that although isotope labeling technology has the characteristics of high precision and high sensitivity, its application is also limited by experimental conditions and analysis techniques. Therefore, in practical application, we need to combine other characterization means and experimental methods to obtain more comprehensive and accurate information to verify the LOM mechanism. In summary, combined with the practical principles of isotope labeling techniques, the use of 18O probes or H isotope probes can more accurately trace the transfer paths of oxygen and hydrogen atoms, thus verifying whether the lattice oxygen is involved in the OER process and further determining the LOM mechanism. This approach provides a powerful tool for in-depth study of the OER reaction mechanism and optimization of electrocatalyst design.
Theoretical calculations used to characterize the LOM
DFT for verifying the LOM mechanism is mainly based on quantum mechanical principles, which reveals the specific mechanism of lattice oxygen's role in the OER process by simulating and calculating the electronic structure of the catalyst and the energy changes during the reaction process[43,96]. DFT is able to accurately describe the inter-atomic interactions and the electronic structure, and thus predicts the activity, selectivity and stability of the catalyst. In practice, the researchers first constructed an atomic model of the catalyst and used the DFT method for geometry optimization and electronic structure analysis to determine the stable configuration and electronic properties of the catalyst. Then, by simulating possible reaction pathways and intermediate states, the energy changes and transition state structures during the reaction process were calculated to reveal the key steps and energy barriers in the LOM mechanism[97,98]. Finally, the results of DFT calculations were compared and verified with experimental results to confirm the correctness and reliability of the LOM mechanism. For example, DFT both simultaneously compared the AEM/LOM catalytic cycle of BM/BiFeOxHy[99] [Figure 5A], B, Fe Co-doped CoP[81] (B,Fe-CoP) [Figure 5B] electrocatalysts, highlighting the LOM in OER activity.
Figure 5. (A) Schematic representation of the adsorption kinetics of BM/BiFeOxHy on LOM compared to conventional AEM (Copyright 2023, Elsevier[99]); (B) Step diagrams of Gibbs free energy maps for the AEM pathway and LOM pathway on CoFe (Copyright 2023, John Wiley and Sons[81]). AEM: Adsorption evolution mechanism; LOM: Lattice oxygen mechanism;
In order to determine the mechanism of electrocatalytic OER, researchers need to construct and simulate the reaction models under different mechanisms through DFT calculations. By calculating key parameters such as energy barriers, reaction rates, electron transfer and distribution in the reaction pathways, we can gain insight into the kinetic and electronic properties of the reactions under various mechanisms. In this step, we will focus on the energy changes and electronic structure changes during the reaction to reveal the reaction mechanism and active sites of the electrocatalysts under different mechanisms. We need to assess the possibility and importance of varying mechanisms, then construct and simulate reaction models for calculations, and finally verify the calculations with experimental data and optimize the theoretical models. In this process, we need to integrate the knowledge and technical tools from multiple disciplines, such as chemistry, physics and computer science, in order to deeply understand the reaction mechanism of electrocatalytic OER and optimize the performance of electrocatalysts.
The catalyst design in an acidic medium should follow the following principles: corrosion resistance. The catalyst material should have good resistance to acid corrosion, and be able to maintain the stability of structure and performance for a long time in an acidic medium. High activity: The electrocatalyst should have high catalytic activity and be able to realize efficient catalytic reaction at low temperature and pressure. High selectivity: The electrocatalyst should be able to selectively promote the target reaction path and inhibit the occurrence of undesired side reactions. Long life: The electrocatalysts should have a long service life and low deactivation rate to meet the requirements of long-term stability and reliability in practical applications. Low cost: The preparation cost of the catalyst should be as low as possible to reduce the overall economic cost of the process. Environmental protection: The use of electrocatalysts should meet the requirements of environmental protection, avoiding the generation of toxic and harmful waste or pollution to the environment.
For the LOM, the detection of lattice oxygen characterization in the electrocatalyst itself is the first step in determining the mechanism. For the characterization and analysis of Lattice Oxygen, several commonly used techniques can be briefly summarised as follows.
Infrared (IR) Spectroscopy: In the characterization of Lattice Oxygen, the lattice oxygen basicity of materials such as protonated zeolites can be investigated, especially by the CO2 probe IR method (FTIR Study of Characterization of Basicity of Lattice Oxygen). For example, the basicity of lattice oxygen in proton zeolites can be characterized by the low-temperature CO2 probe IR method. The method utilizes the interaction of CO2 molecules with lattice oxygen, and the nature and amount of lattice oxygen is inferred from the characteristic absorption peaks of the IR spectrum.
Electron Microscopy: The distribution and state of lattice oxygen in a catalyst or material can be observed by high-resolution imaging and energy spectrum analysis [energy-dispersive X-ray spectroscopy (EDS)]. In electrocatalyst research, EDS can be used to observe phenomena such as defects and reconstruction of lattice oxygen, and their relationship with catalyst performance.
Synchrotron Radiation Techniques: The local coordination environment and electronic structure of lattice oxygen can be studied by techniques such as X-ray absorption fine structure (XAFS). In the study of lattice oxygen in electrocatalysts and materials, Synchrotron Radiation Techniques can be used to accurately determine the oxidation state of lattice oxygen, the coordination number and the bond lengths with the surrounding atoms, which can provide an important basis for understanding its catalytic mechanism.
Mössbauer Spectroscopy: It is a method for studying the microstructure of matter using ultrafine interactions between atomic nuclei and their surroundings. For samples containing specific radioisotopes, Mössbauer Spectroscopy can provide detailed information about the interactions between lattice oxygen and metal ions.
In-situ Characterization Techniques: In-situ characterization techniques are technical means of characterizing a catalyst or material in real time under reaction conditions. These techniques can simulate the actual working conditions and directly observe the structural and property changes of the catalyst during the reaction process.
In summary, there are various methods for the characterization and analysis of lattice oxygen, and each has its unique advantages and scope of application. In practical research, suitable methods can be selected for characterization and analysis according to specific research objects and purposes.
THE CASE OF LOM ELECTROCATALYSTS IN ACIDIC OER
Lattice LOM is emerging as a key strategy to improve electrocatalytic performance in acidic OER and PEMWE[100,101]. LOM electrocatalysts provide a low-energy barrier pathway for O2 generation by activating O atoms in the catalyst lattice. Ir and Ru-based metal oxides have become hot spots in the study of LOM electrocatalysts due to their intrinsic high activity and stability[102-104]. By precisely controlling the synthesis conditions, nanostructures with optimized lattice oxygen activity and abundant oxygen vacancies can be obtained, thus improving the OER performance. Non-precious metal oxides (compounds based on Fe, Co and Ni)[48,105,106] have attracted much attention due to their cost-effectiveness and abundance of earth resources. Through innovative synthesis methods (solvothermal, co-precipitation and template methods), materials with high specific surface area and porous structures can be prepared, thus facilitating ion diffusion and exposure of active sites in the electrolyte. In addition, the electronic properties and LOM activity of these materials can be further optimized by doping with non-metallic elements or forming complex oxides[107,108].
Ir-based electrocatalysts
Ir-based electrocatalysts typically follow the traditional AEM when performing OER in acidic environments[109,110]. However, if Ir-based electrocatalysts can be made to follow the LOM in the OER reaction through different modulation means, the bottleneck of activity deficiency will be broken, but this puts severe demands on the electrocatalysts. Firstly, the LOM mechanism allows Ir-based electrocatalysts to exhibit higher activity and efficiency in the OER process. This is because the LOM mechanism involves the participation of lattice oxygen, which helps promote the O2 dissociation reaction[111]. By providing activation energy, the LOM mechanism is able to accelerate the reaction rate of the OER, thereby increasing the overall efficiency of the water electrolysis unit. Second, the transition to the LOM mechanism improves the stability and durability of Ir-based electrocatalysts[112]. In the AEM mechanism, the electrocatalyst surface may undergo frequent adsorption and desorption processes, leading to catalyst deactivation or degradation. The LOM mechanism reduces such surface changes, thereby extending the electrocatalyst's lifetime[113,114]. To achieve this transition, the requirements for electrocatalysts will increase accordingly. Firstly, the electrocatalyst needs to have the right crystal structure and chemical composition to support the LOM mechanism to occur. This may need to be achieved through fine synthesis and modulation[115-118]. Secondly, the surface properties of the electrocatalysts also need to be optimized to ensure that they can effectively interact with the reactants and facilitate OER. This may involve modulation of the electronic structure of the electrocatalyst surface, active site distribution[119,120]. In addition, in-depth characterization and performance testing of the catalysts is required to verify their successful compliance with the LOM mechanism and to assess their performance in the OER reaction.
Wu et al.[121]. found that the addition of different metals to replace IrO2 resulted in improved activity [Figure 6A]. Among them, the addition of trivalent metals increases Ir-O covalency, which significantly improves the acidic OER activity of rutile IrO2, while the addition of high-valent metals decreases Ir-O covalency, leading to a decrease in OER activity. Experimental and theoretical analyses show that cation substitution enhances the oxygen vacancy (Vo) concentration [Figure 6B] and that the enhancement of Ir-O covalency activates lattice oxygen and triggers a lattice-oxygen-mediated mechanism to enhance the OER kinetics, and that the obtained Gd-IrO2-δ requires only 260 mV overpotential to reach 10 mA cm-2 [Figure 6C]. Wang et al.[122]. reported a cubic fluorite-structured praseodymium-iridium oxide (Pr3IrO7) electrocatalyst containing a highly active surface layer of IrOx. The decisive speed step energy barriers of AEM and LOM were compared by DFT [Figure 6D], and the electrochemical experimental data [Figure 6E] were compared with each other to derive the LOM pathway for the energy-first catalytic reaction and to reveal the catalytic reaction mechanism. Tan et al.[82]. could convert the AEM-dominated OER pathway into the LOM-dominated OER pathway by pre-electrochemical acid etching treatment of a mixture of IrOx and Y2O3 (IrOx/Y2O3) [Figure 6F-H]. Shi et al.[123]. demonstrated that a unit iridium doping strategy effectively triggers lattice oxygen oxidation reductions to improve the kinetics of the OER, essentially an iridium chelating environment, where Ir-O covalency is improved and lattice oxygen oxidation is involved [Figure 6I-K].
Figure 6. (A) Schematic of partial substitution of Ir by Gd, Pr, Nd, Mo and W atoms; (B) O 1s XPS spectra of Gd-IrO2-δ electrocatalyst; (C) OER performance of each electrocatalyst in acidic medium (Copyright 2023, John Wiley and Sons[121]). (D) Acidic OER performance of IrOx/Pr3IrO7 and (E) free energy maps based on AEM and LOM paths (Copyright 2021, Springer Nature[122]); (F) TEM images of IrOx/Y2O3; (G) polarization curves and Tafel slopes of different electrocatalysts; (H) The electrocatalytic OER pathway of iridium oxide converted from AEM to LOM by electrochemical etching pre-treatment (Copyright 2023, John Wiley and Sons[82]). The constructed Ir-MnO2 is theoretically prepared for activity stability (I); and electrochemical tests (J) and DFT (K) corroborate the activity mechanism (Copyright 2021, Elsevier[123]). OER: Oxygen generation reaction; AEM: Adsorption evolution mechanism; LOM: Lattice oxygen mechanism; DFT: Density functional theory; XPS: X-ray photoelectron spectroscopy; TEM: Transmission electron microscope.
In conclusion, the modulation of Ir-based catalysts to follow the LOM mechanism for the OER reaction process needs to be based on the covalency of Ir-O, which is expected to improve the electrocatalyst activity and promote the further development of electrochemical water decomposition technology. However, in order to achieve this goal, in-depth research and optimization of the electrocatalysts are needed to meet the specific requirements of the LOM mechanism for electrocatalysts.
Ru-based electrocatalysts
Ru-based electrocatalysts for OER in acidic environments usually follow the LOM pathway [Figure 7A], which helps the electrocatalysts to exhibit high activity under specific conditions[124,125]. However, the stability problem of Ru-based electrocatalysts in acidic OER has been a major obstacle to their application
Figure 7. (A) AEM and (B) LOM pathways of Ru-based electrocatalysts; (C) Overlap between Ru 4d and the O 2p ligand state during AEM and (D) orbital optimization leading to the LOM: Ru 4d center is downshifted to penetrate the O 2p ligand band, leading to increased filling of the antibonding (eg) state, and modulation of orbital hybridization for increasing the Ru-O covalency, thus activating the participation of lattice O in the OER. (Copyright 2023, American Chemical Society[131]); (E) Schematic representation of the oxidation of RuO2 to a high valence state in the acidic OER of the LOM pathway (upper panel) and the stability of the MOF-anchored Ru intermediate (lower panel); (F) Schematic representation of CV peak intermediates with and without TMA+ and (J) Gibbs free energy step diagrams for Ru-UiO-67-bpydc and RuO2 catalysts during OER via AEM or LOM pathway (Copyright 2023, Elsevier); XRD diagram (H) and HAADF-STEM image (I) of Mn0.73Ru0.27O2-δ and the charge redistribution process (J) in the oxide after the addition of Ru (Copyright 2022, Royal Society of Chemistry); (K) Free energy diagram for OER on Ru1-N4, O-Ru1-N4, and HO-Ru1-N4; (L) Schematic of the whole OER mechanism on Ru-N-C in the acidic electrolyte (Copyright 2019, Springer Nature); (M) Bader charge distribution diagram of RuCoOx and (N) comparison of PDS energy barriers for different reaction pathways; (O) Model of the Ruoct-O-Cooct structure with two adsorbed oxygen radicals (Copyright 2023, American Chemical Society[133]); (P) A two-dimensional activity map for LOM-OVSM mechanism (Copyright 2023, Springer Nature[95]); (Q) O 1s fitting plots for different H-doped RuO2 and (R) the ratio of V-O to O-H in the O 1s fitting results for a series of H-RuO2; (S) Structural maps of the five sites of H in RuO2 and schematic diagrams using the SPE device (Copyright 2022, Elsevier[134]); (T) Schematic structure of LOM converted to AEM in rich grain boundaries; (U) Ru-O elongation as well as electronic shift in GB-RuO2; (V) Comparison of acidic OER stability and activity of RuO2-based catalysts (Copyright 2024, John Wiley and Sons[93]). AEM: Adsorption evolution mechanism; LOM: Lattice oxygen mechanism; OER: Oxygen generation reaction; TMA: TetraMethylAmmonium; MOF: Metal organic framework; XRD: X-ray diffraction; PDS: Potential-determining step; LOM-OVSM: Lattice oxygen mechanism-oxygen vacancy site mechanism.
However, recent works have favored the LOM mechanism for inhibiting the OER reaction of Ru-based electrocatalysts, mainly because the LOM mechanism, although capable of providing high catalytic activity, is often accompanied by poor stability[102,127-129]. In the LOM mechanism, the involvement of lattice oxygen may lead to the destruction of the electrocatalyst structure and inactivation of the active sites, thus reducing the durability of the electrocatalyst[130]. Therefore, in order to overcome this challenge, researchers have started to try to prepare Ru-based electrocatalysts that can follow the AEM or the OPM[42,131], which may be able to improve the stability of the catalysts while maintaining a certain catalytic activity. By carefully designing and adjusting the composition and structure of the electrocatalysts and the reaction conditions, it is expected that a Ru-based electrocatalyst can be found that can obtain high activity and maintain stability in acidic OER. Wang et al.[132]. developed a rutile-structured ruthenium-manganese solid-solution oxide (Mn0.73Ru0.27O2-δ) [Figure 7H-I] with oxygen vacancies, which was an electrocatalyst reflecting pH-independent properties, and the OER followed the AEM pathway, and the introduction of the oxygen vacancies could further promote the electron transfer between OOH* intermediates [Figure 7J] and the active Ru sites on the surface of (211) surface-active Ru sites, thereby accelerating the adsorption kinetics and enhancing the OER activity [Figure 7K and L]. Zhu et al.[133]. prepared a novel RuCoOx electrocatalyst by introducing Ru atoms into the octahedral positions of spinel-type Co3O4, replacing part of the Co atoms [Figure 7K]. The Ru-O-Co coordination structure exhibits a strong electronic coupling, which facilitates the catalyst to undergo a thermodynamically more favorable novel heterogeneous lattice oxygen-mediated pathway (DOM, Figure 7M-O) via the OER. Wang et al.[95]. demonstrated a synergistic modulation strategy in terms of low-valent Rh-doped RuO2 (Rh-RuO2/G) electrocatalysts, which opens up a completely new LOM-oxygen vacancy site mechanism (OVSM) pathway for obtaining OERs with both activity and stability in acidic media [Figure 7P]. Besides proposing to testify that the electrocatalysts follow some new acidic OER mechanisms, the researchers moreover try some strategies to stabilize the lattice oxygen, the dominant catalyst in suppressing the OER on the LOM pathway. He et al.[134]. then proposed a proton and electron co-doping strategy to achieve hydrogen-doped (H-doping) RuO2 and prevented the evolution of lattice oxygen [Figure 7Q-R], which enhanced the stability and resulted in excellent stability during SPE water electrolysis [Figure 7S]. He et al.[93]. proposed a grain boundary (GB) engineering strategy that prevented the dissolution of Ru and greatly suppressed the lattice LOM [Figure 7T-V].
The LOM mechanism, which involves the participation of lattice oxygen in the treatment of acidic OERs, may lead to the destruction of the electrocatalyst structure or the loss of active components for Ru-based catalysts with poor ability to resist electrochemical corrosion, thus affecting their long-term stability. In contrast, the structure of Ru-based electrocatalysts can be better stabilized and their durability can be enhanced by optimizing their structure, improving the synthesis method and exploring new reaction mechanisms.
Non-precious metal-based electrocatalysts
The modulation of non-precious metal oxides helps reduce the cost of electrocatalysts[135-137]. Since non-precious metals are abundant and relatively inexpensive in the earth's crust, the use of such catalysts can greatly reduce the economic threshold of renewable energy technologies such as hydrogen production from electrolytic water, thus promoting their widespread application[137]. In addition, the activity and stability of the electrocatalysts can be improved by modulating the non-precious metal oxides to follow the LOM mechanism in the acidic OER reaction, which helps enhance the interaction between the catalysts and the reactants, accelerate the electron transfer and the release of O2, and thus improve the efficiency of the OER reaction and the energy conversion efficiency[138].
The modulation of non-precious metal oxides usually involves precise control of catalyst composition, structure, morphology and surface properties. Non-noble metal oxide catalysts with specific crystal structure, pore structure and surface chemistry can be prepared by using advanced synthetic methods[139,140]. Meanwhile, the electronic structure and catalytic activity of the catalysts can be further regulated by doping and surface modification to obtain better LOM catalytic performance[141]. From the research progress, scientists have made remarkable breakthroughs in LOM catalysis OER using non-precious metal oxides as catalysts in recent years[73]. On the one hand, through the in-depth study of the catalytic mechanism of non-precious metal oxides, the key sites and reaction pathways in the LOM mechanism have been revealed, which provides a theoretical basis for the design and optimization of catalysts. On the other hand, a series of highly efficient and stable non-precious metal oxide catalysts have been successfully prepared through the development of new synthesis techniques and regulatory strategies, which exhibit excellent LOM catalytic performance in acidic OER reactions. Yang et al.[73]. prepared N-doped 3D Co3O4/NC-250 electrocatalysts using an innovative carbon-oxidation process, which exhibited excellent acidic OER performance due to the abundance of oxygen vacancies [Figure 8A-B]. The in situ ATR-FTIR observation of the intermediate O*O provides direct evidence for the LOM mechanism. DFT calculations further support the contribution of the Vo-facilitated LOM mechanism in lowering the energy barrier at the critical step of the OER process
Figure 8. O 1s XPS (A) and EPR (B) spectra of 2D/3D Co3O4/NC-250; (C) ΔGH of adsorption at Co active sites by the AEM mechanism for 2D/3D Co3O4/NC-250; (D) OER step diagram of the AEM and LOM mechanisms at the 3D Co3O4/NC Ov active site (Copyright 2023, Elsevier[73]); (E) XANES and k3-weighted Fourier-transformed EXAFS spectra of MnO2 with different cations and the K+/MnO2 structure and Co2+/MnO2 structure after the substitution, In situ Raman spectra of 18O-labeled Co2+/MnO2 in (F) Mn-O and (G)*OO2- region; (H) The main OER mechanism of Co2+/MnO2 (Copyright 2023, Royal Society of Chemistry[142]); (E) TEM image, HR-TEM image (I) and AC-HAADF-STEM image, atomic profiles (J) of CoSA-MoCeOx@BCT; In situ Raman spectra (K), OER performance (L) and LOM catalytic cycle (M) of CoSA-MoCeOx@BCT (Copyright 2024, Royal Society of Chemistry[105]). AEM: Adsorption evolution mechanism; LOM: Lattice oxygen mechanism; OER: Oxygen generation reaction; XPS: X-ray photoelectron spectroscopy; EPR: Electron paramagnetic resonance; XANES: X-ray absorption near edge structure.
The modulation of non-precious metal oxides in acidic OER reaction catalyzed by LOM mechanism has significant economic benefits and environmental significance, and its unique modulation characteristics and important research progress provide new ideas and methods for the design and optimization of catalysts. With the continuous progress of science and technology, it is believed that more remarkable results and applications of non-precious metal oxides in LOM-catalyzed OER will be achieved in the future.
OER in acidic environments has made remarkable progress in the field of materials science [Table 1]. The Ir/Ru-based electrocatalysts have demonstrated excellent catalytic performance through fine structural design and component modulation, while the non-precious metal oxide electrocatalysts have gradually narrowed the gap with the precious metal electrocatalysts through innovative design and modification strategies. In the future, with the deepening of the research and the emergence of new materials, it is believed that the performance of acidic OER electrocatalysts will be further improved, which will provide strong support for the commercial application of PEMWE technology.
Reported performance and stability of Electrocatalysts for OER reactions involving lattice oxygen in acidic media
| Electrocatalyst | electrolyte | Overpotential (mV @η10) | Tafel slope (mV dec-1) | Stability | Ref. |
| Gd-IrO2–δ | 0.5 M H2SO4 | 260 | 55.4 | ~ 200 h | [121] |
| IrOx/Pr3IrO7 | 0.1 M HClO4 | 305 | 37 | > 16 h | [122] |
| Ir–MnO2 | 0.5 M H2SO4 | 218 | 59.61 | > 600 h | [123] |
| IrOx/Y2O3 | 0.5 M H2SO4 | 257 | 61.3 | > 200 h | [82] |
| Ir/Nb2O5−x | 0.5 M H2SO4 | 218 | 52.3 | > 100 h | [143] |
| Ru array-Co3O4 | 0.5 M H2SO4 | 160 | 46 | ~ 1500 h | [131] |
| Mn0.73Ru0.27O2−δ | 0.5 M H2SO4 | 208 | 65.3 | ~ 10 h | [132] |
| Ru/MnO2 | 0.1 M HClO4 | 160 | 29.4 | 200 h | [42] |
| Ru1–Pt3Cu | 0.1 M HClO4 | 220 | - | > 28 h | [144] |
| Ru-N-C | 0.5 M H2SO4 | 267 | 52.6 | > 30 h | [145] |
| 3D Co3O4/NC | 0.5 M H2SO4 | 270 | 124 | 80 h | [73] |
| CoSA-MoCeOx@BCT | 0.5 M H2SO4 | 239 | 29.0 | 60 h | [105] |
In order to improve the stability of catalysts in acidic OER, we summarize the following strategies and design principles. Optimization of lattice oxygen activity and stability: We discuss the enhancement of OER performance by modulating the activity of lattice oxygen while maintaining the stability of the catalyst structure. This includes optimizing the electronic structure of the lattice oxygen by doping, heterogeneous atom substitution or constructing composite oxides with specific lattice oxygen configurations. Building corrosion-resistant surface structures: We emphasize the importance of designing surface structures that are resistant to corrosion by acidic media. This may involve the use of materials with high corrosion resistance, such as Ir- and Ru-based oxides, or surface modifications to enhance their stability in acidic environments. Tuning the electronic structure: We propose to improve the stability of catalysts in acidic media by tuning their electronic structure, and by optimizing the electron transfer process by modulating the band gap, Fermi energy levels and energy band structure. Utilizing protective layers or coatings: We explore the possibility of introducing protective layers or coatings on the catalyst surface to isolate the catalyst from direct contact with acidic media and thus reduce corrosion. Designing porous or multiphase structures: We discuss the role of porous or multiphase structures in improving the stability of catalysts by providing more active sites while improving the overall structural stability by dispersing stresses. Exploitation of lattice defects: We propose the use of lattice defects, such as oxygen vacancies, to modulate the electronic properties and chemical stability of catalysts. Oxygen vacancies can act as active sites while enhancing the stability of the structure by modulating the distribution of lattice oxygen. Dynamic structural tuning: We discuss the dynamic structural adjustments that catalysts may undergo during the OER process and how these changes can be accommodated by designing catalysts with reversible structural changes to maintain long-term stability. Combination of experiment and theory: We highlight the importance of combining experimental observations and theoretical calculations to understand the mechanisms of catalyst stability in acidic media, which can help to predict and optimize catalyst performance. Long-term stability tests: We recommend long-term stability testing to assess the performance and durability of catalysts under simulated real-world application conditions. Through these strategies and design principles, we aim to improve the stability of catalysts in acidic OERs, thereby advancing their use in practical energy conversion and storage technologies.
PERSPECTIVE AND CONCLUSION
Outlook
With a deeper understanding of the role of lattice LOM in acidic OER, the focus of future research will shift towards addressing the current challenges and further optimizing the performance of LOM electrocatalysts. Firstly, new synthesis strategies need to be developed to enable precise control of the catalyst structure and composition in order to improve lattice oxygen activity and stability. Second, an in-depth study of the relationship between electronic structure and LOM activity will help to design catalysts with higher electronic efficiency. In addition, exploring different non-precious metal oxides as LOM electrocatalysts will help reduce costs and improve the availability of materials. Finally, the combination of theoretical calculations and experimental studies will further reveal the OER kinetics in the LOM mechanism and provide theoretical guidance for catalyst optimization.
Metal oxide (Ir/Ru oxide) active fractions or non-precious metal/non-precious metal-precious metal active fractions are the focus of LOM-driven studies in acidic OER, and understanding the relationship between M-O in metal oxides and the LOM mechanism will be an important guideline for the future construction of LOM electrocatalysts with high activity stability.
M-O affects the stability of electrocatalysts
Strength of M-O bond: Strong M-O bond means that the metal ions in the catalyst are more tightly bound to the oxygen ions and are less likely to break during the reaction, thus improving the stability of the catalyst. Conversely, weaker M-O bonds may lead to rapid deactivation of the catalyst during the reaction.
Oxidation of lattice oxygen: In the LOM mechanism, lattice oxygen is oxidized under the driving force of electric potential and participates in the OER reaction to produce oxygen. If the M-O bond is too strong, lattice oxygen is difficult to oxidize and participate in the reaction, thus limiting the catalyst activity. On the contrary, a moderate M-O bond enables the lattice oxygen to be oxidized while maintaining the stability of the catalyst structure.
M-O affects the performance of electrocatalysts
Reactivity: When the energy of M-O bond is moderate, the catalyst shows high activity in the OER reaction. This is because the moderate M-O bond energy makes the lattice oxygen easy to oxidize under the potential and participate in the O-O bond coupling step of the OER reaction, thus increasing the reaction rate.
Selectivity: When the dissociation energy is moderate, the catalyst can give the right amount of oxygen to participate in the reaction without leading to excessive oxidation, thus improving the selectivity of the reaction.
In the future, the following strategies can be referred to in balancing the lattice oxygen activity with the long-term stability of electrocatalysts [Figure 9].
Figure 9. Strategies for balancing lattice oxygen activity and long-term stability of electrocatalysts.
Structural design and regulation of electrocatalysts.
Specific crystal surface exposure: By precisely controlling the synthesis conditions, the electrocatalysts are exposed to specific crystal surfaces with higher activity. The stability of different crystalline surfaces in acidic environments varies, and the selection and optimization of these surfaces can significantly enhance the catalytic activity and durability of the electrocatalysts.
Construct a core-shell structure: Construct a core-shell structure in which the core is a highly active metal and the shell is a protective or co-catalyst material. This structure can maintain high activity and prevent the degradation of the core metal in acidic environments through the shell layer material, thus increasing the overall stability.
Alloying and doping: Forming alloys of noble metals with other metals or doping trace transition metals can adjust the electronic structure of the electrocatalysts and enhance their activity and durability.
Carrier selection and interface modulation
Mixed metal oxide carriers: the active components (Ir/Ru) are anchored on mixed metal oxide carriers, and the oxygen diffusion pathways are constructed through interfacial modulation. This strategy can enhance the activity while protecting the carrier from excessive oxidation, thus improving the overall stability of the catalyst.
Interfacial construction: Use two- or three-dimensional nanomaterials to construct composite catalysts, and increase the number of active sites and improve the catalytic efficiency and stability by regulating the contact mode and interfacial construction between different materials.
Innovation of catalyst preparation methods
High-temperature quenching technology: High-temperature quenching technology, such as flame spraying method, is used to ensure the formation of oxide crystals while inhibiting their atomic rearrangement process and locking the lattice oxygen in a sub-stable state of oversaturation. This method increases the disorder of lattice oxygen and improves the desorption activity while maintaining the stability of the catalyst.
Microstructure modulation: the stability of the catalyst is improved by means of microstructure modulation such as designing lattice defects and regulating the mobility of the catalyst. For example, an appropriate amount of oxygen atom doping can reduce the specific surface area of the catalyst and active site density, thus prolonging the service life of the catalyst.
Optimization of reaction conditions
Reaction environment control: By controlling the acidity and alkalinity of the reaction environment and selecting suitable solvents and additives, the nature of the active sites of the catalyst can be affected, which, in turn, changes the catalytic performance.
Heat treatment and post-treatment: In the process of catalyst preparation, appropriate heat treatment or post-treatment steps, such as high-temperature annealing, vacuum treatment, etc., can further adjust the microstructure and surface properties of the catalyst and improve its stability and activity.
Combination of theoretical calculation and experimental verification
DFT: Using DFT and other theoretical calculations, we can deeply investigate the AEM and electronic structure changes of the catalyst, and provide theoretical guidance for the design and optimization of the catalyst.
In-situ DEMS analysis: through in-situ DEMS and other experimental means, real-time monitoring of the catalyst changes during the reaction process, verifying the theoretical predictions and further optimizing the performance of the catalyst.
Balancing the trade-off between enhancing lattice oxygen activity and ensuring the long-term stability of LOM electrocatalysts in acidic environments is a complex and important task. The catalytic activity and durability of the catalysts can be significantly enhanced through the combined application of various strategies, such as the structural design and regulation of the catalysts, carrier selection and interfacial regulation, innovation of the preparation method, optimization of the reaction conditions, and the combination of theoretical calculations and experimental validation, which can provide a strong guarantee of an efficient and stable catalytic process.
Conclusion
This paper reviewed the current status of LOM application in acidic OER, analyzed the difference between LOM and traditional AEM mechanisms, and discussed the validation method of LOM mechanisms. It is shown that the LOM mechanism provides a new way to design efficient and stable OER electrocatalysts. The performance of acidic OER can be significantly improved by precisely modulating the composition and structure of the catalyst. Despite the challenges of stability and active site modulation, the OER electrocatalysts under the LOM mechanism are expected to achieve large-scale applications in the future through continuous material innovation and mechanism studies. In addition, LOM modulation of non-precious metal oxides offers new possibilities for low-cost and efficient conversion of renewable energy technologies. Therefore, in-depth research and application development of the LOM mechanism will play a key role in promoting the development of energy conversion and storage technologies.
Facing the transformation of global energy structure and environmental challenges, the study of the LOM mechanism in acid OER has not only theoretical significance but also practical application value. Through the review in this thesis, we expect to provide new research ideas for researchers, promote the development of LOM electrocatalysts, and contribute to the progress of clean energy technologies. In the future, we expect to see more innovative research results in this field, realizing the transition from laboratory research to industrial applications, and contributing to building a sustainable energy future.
DECLARATIONS
Authors’ contributions
Designing research, writing the paper: Xie, Y.
Data visualization, funding acquisition: Luo, F.
Review, supervision: Yang, Z.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work is supported by the National Natural Science Foundation of China (No. 22209126).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Long, X.; Tang, W.; Li, C.; et al. A superior zinc-air battery performance achieved by CoO/Fe3O4 heterostructured nanosheets. Chem. Commun. 2024, 60, 5747-50.
2. Luo, F.; Yu, Y.; Liu, X.; Xie, Y.; Yang, Z. Interfacial electronic modulation enables a robust methanol oxidation. Int. J. Hydrogen. Energy. 2024, 59, 369-74.
4. Han, M.; Mu, Y.; Wei, L.; Zeng, L.; Zhao, T. Multilevel carbon architecture of subnanoscopic silicon for fast-charging high-energy-density lithium-ion batteries. Carbon. Energy. 2024, 6, e377.
5. Pan, S.; Yang, Z.; Luo, F. Ni-based electrocatalysts for urea-assisted water splitting. Chinese. J. Struct. Chem. 2024, 43, 100373.
6. Xu, X.; Sun, H.; Jiang, S. P.; Shao, Z. Modulating metal-organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. SusMat 2021, 1, 460-81.
7. Li, Z.; Wu, X.; Jiang, X.; et al. Surface carbon layer controllable Ni3Fe particles confined in hierarchical N-doped carbon framework boosting oxygen evolution reaction. Adv. Powder. Mater. 2022, 1, 100020.
8. Ding, S.; Wang, H.; Dai, X.; et al. Mn-modulated Co-N-C oxygen electrocatalysts for robust and temperature-adaptative zinc-air batteries. Chinese. J. Struct. Chem. 2024, 43, 100302.
9. Long, X.; Xiong, T.; Bao, H.; et al. Tip and heterogeneous effects co-contribute to a boosted performance and stability in zinc air battery. J. Colloid. Interface. Sci. 2024, 662, 676-85.
10. Xu, B.; Ouyang, T.; Wang, Y.; et al. Progresses on two-phase modeling of proton exchange membrane water electrolyzer. Energy. Rev. 2024, 3, 100073.
11. Deng, Y.; Luo, J.; Chi, B.; et al. Advanced atomically dispersed metal-nitrogen-carbon catalysts toward cathodic oxygen reduction in PEM fuel cells. Adv. Energy. Mater. 2021, 11, 2101222.
12. Yeo, K.; Kim, H.; Lee, K.; et al. Controlled doping of ultralow amounts Ru on Ni cathode for PEMWE: experimental and theoretical elucidation of enhanced performance. Appl. Catal. B. 2024, 346, 123738.
13. Amano, F.; Tsushiro, K. Proton exchange membrane photoelectrochemical cell for water splitting under vapor feeding. Energy. Mater. 2024, 4, 400006.
14. Li, S.; Jiang, J.; Zhai, N.; et al. A half-wave rectifying triboelectric nanogenerator for self-powered water splitting towards hydrogen production. Nano. Energy. 2022, 93, 106870.
15. Chen, X.; Yan, Z.; Yu, M.; et al. Spinel oxide nanoparticles embedded in nitrogen-doped carbon nanofibers as a robust and self-standing bifunctional oxygen cathode for Zn-air batteries. J. Mater. Chem. A. 2019, 7, 24868-76.
16. Zhang, L.; Jang, H.; Liu, H.; et al. Sodium-decorated amorphous/crystalline RuO2 with rich oxygen vacancies: a robust pH-universal oxygen evolution electrocatalyst. Angew. Chem. Int. Ed. Engl. 2021, 60, 18821-9.
17. Reier, T.; Nong, H. N.; Teschner, D.; Schlögl, R.; Strasser, P. Electrocatalytic oxygen evolution reaction in acidic environments - reaction mechanisms and catalysts. Adv. Energy. Mater. 2017, 7, 1601275.
18. Chen, Y.; Shang, C.; Xiao, X.; Guo, W.; Xu, Q. Recent progress of electrocatalysts for acidic oxygen evolution reaction. Coord. Chem. Rev. 2024, 508, 215758.
19. Chen, Z.; Fan, Q.; Zhou, J.; et al. Toward understanding the formation mechanism and OER catalytic mechanism of hydroxides by in situ and operando techniques. Angew. Chem. Int. Ed. Engl. 2023, 62, e202309293.
20. Wang, Z.; Goddard, W. A.; Xiao, H. Potential-dependent transition of reaction mechanisms for oxygen evolution on layered double hydroxides. Nat. Commun. 2023, 14, 4228.
21. Song, H.; Yong, X.; Waterhouse, G. I.; et al. RuO2-CeO2 lattice matching strategy enables robust water oxidation electrocatalysis in acidic media via two distinct oxygen evolution mechanisms. ACS. Catal. 2024, 14, 3298-307.
22. Wang, N.; Ou, P.; Miao, R. K.; et al. Doping shortens the metal/metal distance and promotes OH coverage in non-noble acidic oxygen evolution reaction catalysts. J. Am. Chem. Soc. 2023, 145, 7829-36.
23. Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv. Mater. 2021, 33, e2004243.
24. Xie, Y.; Yang, Z. Morphological and coordination modulations in iridium electrocatalyst for robust and stable acidic OER catalysis. Chem. Rec. 2023, 23, e202300129.
25. Siwal, S. S.; Yang, W.; Zhang, Q. Recent progress of precious-metal-free electrocatalysts for efficient water oxidation in acidic media. J. Energy. Chem. 2020, 51, 113-33.
26. Feng, Z.; Dai, C.; Shi, P.; et al. Seven mechanisms of oxygen evolution reaction proposed recently: a mini review. Chem. Eng. J. 2024, 485, 149992.
27. Zhang, Q.; Xiao, W.; Fu, H. C.; et al. Unraveling the mechanism of self-repair of NiFe-based electrocatalysts by dynamic exchange of iron during the oxygen evolution reaction. ACS. Catal. 2023, 13, 14975-86.
28. Lazaridou, A.; Smith, L. R.; Pattisson, S.; et al. Recognizing the best catalyst for a reaction. Nat. Rev. Chem. 2023, 7, 287-95.
29. Sun, L.; Feng, M.; Peng, Y.; et al. Constructing oxygen vacancies by doping Mo into spinel Co3O4 to trigger a fast oxide path mechanism for acidic oxygen evolution reaction. J. Mater. Chem. A. 2024, 12, 8796-804.
30. Wang, D.; Xue, J.; Ding, X.; et al. Neighboring cationic vacancy assisted adsorption optimization on single-atom sites for improved oxygen evolution. ACS. Catal. 2022, 12, 12458-68.
31. Jiao, Y.; Yan, H.; Tian, C.; Fu, H. Structure engineering and electronic modulation of transition metal interstitial compounds for electrocatalytic water splitting. Acc. Mater. Res. 2023, 4, 42-56.
32. Kaushik, S.; Xiao, X.; Xu, Q. Design strategies of electrocatalysts for acidic oxygen evolution reaction. EnergyChem 2023, 5, 100104.
33. Pham, C. V.; Escalera-lópez, D.; Mayrhofer, K.; Cherevko, S.; Thiele, S. Essentials of high performance water electrolyzers - from catalyst layer materials to electrode engineering. Adv. Energy. Mater. 2021, 11, 2101998.
34. Huang, Z.; Song, J.; Du, Y.; et al. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy. 2019, 4, 329-38.
35. Wang, Y.; Hao, S.; Liu, X.; et al. Ce-doped IrO2 electrocatalysts with enhanced performance for water oxidation in acidic media. ACS. Appl. Mater. Interfaces. 2020, 12, 37006-12.
36. Xie, Y.; Feng, Y.; Pan, S.; et al. Electrochemical leaching of Ni dopants in IrRu alloy electrocatalyst boosts overall water splitting. Adv. Funct. Mater. 2024, 34, 2406351.
37. Grimaud, A.; Demortière, A.; Saubanère, M.; et al. Activation of surface oxygen sites on an iridium-based model catalyst for the oxygen evolution reaction. Nat. Energy. 2017, 2, 16189.
38. Zhou, D.; Li, P.; Lin, X.; et al. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50, 8790-817.
39. Zhou, L.; Lu, S.; Guo, S. Recent progress on precious metal single atom materials for water splitting catalysis. SusMat 2021, 1, 194-210.
40. Rong, C.; Dastafkan, K.; Wang, Y.; Zhao, C. Breaking the activity and stability bottlenecks of electrocatalysts for oxygen evolution reactions in acids. Adv. Mater. 2023, 35, e2211884.
41. Yuan, C.; Zhao, H.; Huang, S.; et al. Designing and regulating catalysts for enhanced oxygen evolution in acid electrolytes. Carbon. Neutralization. 2023, 2, 467-93.
42. Lin, C.; Li, J.; Li, X.; et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 2021, 4, 1012-23.
43. Zhang, N.; Feng, X.; Rao, D.; et al. Lattice oxygen activation enabled by high-valence metal sites for enhanced water oxidation. Nat. Commun. 2020, 11, 4066.
44. Du, H.; Luo, H.; Jiang, M.; Yan, X.; Jiang, F.; Chen, H. A review of activating lattice oxygen of metal oxides for catalytic reactions: reaction mechanisms, modulation strategies of activity and their practical applications. Appl. Catal. A. 2023, 664, 119348.
45. Huang, Z. F.; Xi, S.; Song, J.; et al. Tuning of lattice oxygen reactivity and scaling relation to construct better oxygen evolution electrocatalyst. Nat. Commun. 2021, 12, 3992.
46. Pan, Y.; Xu, X.; Zhong, Y.; et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 2020, 11, 2002.
47. Chen, H.; Lim, C.; Zhou, M.; et al. Activating lattice oxygen in perovskite oxide by B-site cation doping for modulated stability and activity at elevated temperatures. Adv. Sci. 2021, 8, e2102713.
48. Grimaud, A.; Diaz-Morales, O.; Han, B.; et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457-65.
49. Chen, L.; Liu, F.; Li, X.; et al. Surface adsorbed and lattice oxygen activated by the CeO2/Co3O4 interface for enhancive catalytic soot combustion: experimental and theoretical investigations. J. Colloid. Interface. Sci. 2023, 638, 109-22.
50. Zhang, N.; Chai, Y. Lattice oxygen redox chemistry in solid-state electrocatalysts for water oxidation. Energy. Environ. Sci. 2021, 14, 4647-71.
51. Zhai, P.; Wang, C.; Zhao, Y.; et al. Regulating electronic states of nitride/hydroxide to accelerate kinetics for oxygen evolution at large current density. Nat. Commun. 2023, 14, 1873.
52. Jing, C.; Li, L.; Chin, Y. Y.; et al. Balance between FeIV-NiIV synergy and lattice oxygen contribution for accelerating water oxidation. ACS. Nano. 2024, 18, 14496-506.[DOI:10.1021/acsnano.4c01718.
53. Adamu, H.; Abba, S. I.; Anyin, P. B.; Sani, Y.; Yamani, Z. H.; Qamar, M. Tuning OER Electrocatalysts toward LOM pathway through the lens of multi-descriptor feature selection by artificial intelligence-based approach. ACS. Mater. Lett. 2023, 5, 299-320.
54. Zheng, Z.; Wu, D.; Chen, G.; et al. Microcrystallization and lattice contraction of NiFe LDHs for enhancing water electrocatalytic oxidation. Carbon. Energy. 2022, 4, 901-13.
55. Yang, S.; Qin, L.; Zhang, W.; et al. The mechanism of water oxidation from Mn-based heterogeneous electrocatalysts. Chinese. J. Struct. Chem. 2022, 41, 2204022-33.
56. Zhang, G.; Pei, J.; Wang, Y.; et al. Selective activation of lattice oxygen site through coordination engineering to boost the activity and stability of oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2024, 63, e202407509.
57. Han, J.; Wang, H.; Wang, Y.; et al. Lattice oxygen activation through deep oxidation of Co4N by jahn-teller-active dopants for improved electrocatalytic oxygen evolution. Angew. Chem. Int. Ed. Engl. 2024, 63, e202405839.
58. Zhang, Y.; Zhang, W.; Zhang, X.; et al. Activating lattice oxygen based on energy band engineering in oxides for industrial water/saline oxidation. Energy. Environ. Sci. 2024, 17, 3347-57.
59. Li, X.; Cheng, Z.; Wang, X. Understanding the mechanism of the oxygen evolution reaction with consideration of Spin. Electrochem. Energ. Rev. 2021, 4, 136-45.
60. Dong, J.; Qian, Z.; Xu, P.; et al. In situ raman spectroscopy reveals the structure evolution and lattice oxygen reaction pathway induced by the crystalline-amorphous heterojunction for water oxidation. Chem. Sci. 2022, 13, 5639-49.
61. Ma, Q.; Mu, S. Acidic oxygen evolution reaction: mechanism, catalyst classification, and enhancement strategies. Interdiscip. Mater. 2023, 2, 53-90.
62. Hu, Y.; Zheng, Y.; Jin, J.; et al. Understanding the sulphur-oxygen exchange process of metal sulphides prior to oxygen evolution reaction. Nat. Commun. 2023, 14, 1949.
63. Dionigi, F.; Zeng, Z.; Sinev, I.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522.
64. Cho, K. H.; Park, S.; Seo, H.; et al. Capturing manganese oxide intermediates in electrochemical water oxidation at neutral pH by in situ raman spectroscopy. Angew. Chem. Int. Ed. Engl. 2021, 60, 4673-81.
65. Chen, D.; Yu, R.; Yu, K.; et al. Bicontinuous RuO2 nanoreactors for acidic water oxidation. Nat. Commun. 2024, 15, 3928.
66. Hao, Y.; Cao, X.; Lei, C.; Chen, Z.; Yang, X.; Gong, M. Chemical oxygen species on electrocatalytic materials during oxygen evolution reaction. Mater. Today. Catal. 2023, 2, 100012.
67. Su, H.; Zhou, W.; Zhou, W.; et al. In-situ spectroscopic observation of dynamic-coupling oxygen on atomically dispersed iridium electrocatalyst for acidic water oxidation. Nat. Commun. 2021, 12, 6118.
68. Li, N.; Cai, L.; Gao, G.; et al. Operando direct observation of stable water-oxidation intermediates on Ca2-xIrO4 nanocrystals for efficient acidic oxygen evolution. Nano. Lett. 2022, 22, 6988-96.
69. Pastor, E.; Lian, Z.; Xia, L.; et al. Complementary probes for the electrochemical interface. Nat. Rev. Chem. 2024, 8, 159-78.
70. Zhu, K.; Zhu, X.; Yang, W. Application of in situ techniques for the characterization of NiFe-based oxygen evolution reaction (OER) electrocatalysts. Angew. Chem. Int. Ed. Engl. 2019, 58, 1252-65.
71. Xin, S.; Tang, Y.; Jia, B.; et al. Coupling adsorbed evolution and lattice oxygen mechanism in Fe-Co(OH)2/Fe2O 3 heterostructure for enhanced electrochemical water oxidation. Adv. Funct. Materials. 2023, 33, 2305243.
72. Yao, N.; Jia, H.; Zhu, J.; et al. Atomically dispersed Ru oxide catalyst with lattice oxygen participation for efficient acidic water oxidation. Chem 2023, 9, 1882-96.
73. Yang, X.; Cheng, J.; Li, H.; Xu, Y.; Tu, W.; Zhou, J. Self-supported N-doped hierarchical Co3O4 electrocatalyst with abundant oxygen vacancies for acidic water oxidation. Chemical. Engineering. Journal. 2023, 465, 142745.
74. He, Z.; Zhang, J.; Gong, Z.; et al. Activating lattice oxygen in NiFe-based (oxy)hydroxide for water electrolysis. Nat. Commun. 2022, 13, 2191.
75. Nguyen, H. M.; Tang, H.; Huang, W.; Lin, M. Mechanisms for reactions of trimethylaluminum with molecular oxygen and water. Comput. Theor. Chem. 2014, 1035, 39-43.
76. Wang, X.; Xi, S.; Huang, P.; et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature 2022, 611, 702-8.
77. Lim, C. Y. J.; Yilmaz, M.; Arce-Ramos, J. M.; et al. Surface charge as activity descriptors for electrochemical CO2 reduction to multi-carbon products on organic-functionalised Cu. Nat. Commun. 2023, 14, 335.
78. Kamar, R.; Agoston, R.; van, R. G. A.; Hinsley, G.; O'mullane, A. P.; Jones, M. W. M. Probing the effect of metal to ligand charge transfer on the oxygen evolution reaction in Au incorporated Co(OH)2 thin film electrocatalysts. J. Mater. Chem. A. 2023, 11, 20816-23.
79. Chen, F.; Wu, Z.; Adler, Z.; Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704-31.
80. Giordano, L.; Han, B.; Risch, M.; et al. pH dependence of OER activity of oxides: current and future perspectives. Catal. Today. 2016, 262, 2-10.
81. Pan, Y.; Wang, Z.; Wang, K.; et al. Dual doping of B and Fe activated lattice oxygen participation for enhanced oxygen evolution reaction activity in alkaline freshwater and seawater. Adv. Funct. Mater. 2024, 34, 2402264.
82. Tan, X.; Zhang, M.; Chen, D.; et al. Electrochemical etching switches electrocatalytic oxygen evolution pathway of IrOx/Y2O3 from adsorbate evolution mechanism to lattice-oxygen-mediated mechanism. Small 2023, 19, e2303249.
83. Niu, Z.; Lu, Z.; Qiao, Z.; et al. Robust Ru-VO2 bifunctional catalysts for all-pH overall water splitting. Adv. Mater. 2024, 36, e2310690.
84. Han, N.; Zhao, F.; Li, Y. Ultrathin nickel-iron layered double hydroxide nanosheets intercalated with molybdate anions for electrocatalytic water oxidation. J. Mater. Chem. A. 2015, 3, 16348-53.
85. Seabold, J. A.; Choi, K. S. Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode coupled with an iron oxyhydroxide oxygen evolution catalyst. J. Am. Chem. Soc. 2012, 134, 2186-92.
86. Ping, X.; Liu, Y.; Zheng, L.; et al. Locking the lattice oxygen in RuO2 to stabilize highly active Ru sites in acidic water oxidation. Nat. Commun. 2024, 15, 2501.
87. Lagunov, O.; Drenchev, N.; Chakarova, K.; Panayotov, D.; Hadjiivanov, K. Isotopic labelling in vibrational spectroscopy: a technique to decipher the structure of surface species. Top. Catal. 2017, 60, 1486-95.
88. Roth, J. P. Oxygen isotope effects as probes of electron transfer mechanisms and structures of activated O2. Acc. Chem. Res. 2009, 42, 399-408.
89. Kajihara, K.; Skuja, L.; Hosono, H. 18O-labeled interstitial oxygen molecules as probes to study reactions involving oxygen-related species in amorphous SiO2. J. Non-Cryst. Solids. 2012, 358, 3524-30.
90. Wen, X.; Sun, X.; Zhang, S.; Yu, G.; Sargent, S. D.; Lee, X. Continuous measurement of water vapor D/H and 18O/16O isotope ratios in the atmosphere. J. Hydrol. 2008, 349, 489-500.
91. Kerstel, E. R.; van, T. R.; Reuss, J.; Meijer, H. A. Simultaneous determination of the 2H/1H, 17O/16O, and 18O/16O isotope abundance ratios in water by means of laser spectrometry. Anal. Chem. 1999, 71, 5297-303.
92. Fan, J.; Zhang, X.; Han, M.; et al. Amorphous Ni-Fe-Mo oxides coupled with crystalline metallic domains for enhanced electrocatalytic oxygen evolution by promoted lattice-oxygen participation. Small 2024, 20, e2303927.
93. He, W.; Tan, X.; Guo, Y.; Xiao, Y.; Cui, H.; Wang, C. Grain-boundary-rich RuO2 porous nanosheet for efficient and stable acidic water oxidation. Angew. Chem. Int. Ed. Engl. 2024, 63, e202405798.
94. Zhang, L.; Wang, L.; Wen, Y.; Ni, F.; Zhang, B.; Peng, H. Boosting neutral water oxidation through surface oxygen modulation. Adv. Mater. 2020, 32, e2002297.
95. Wang, Y.; Yang, R.; Ding, Y.; et al. Unraveling oxygen vacancy site mechanism of Rh-doped RuO2 catalyst for long-lasting acidic water oxidation. Nat. Commun. 2023, 14, 1412.
96. Zhu, Y.; Tahini, H. A.; Hu, Z.; et al. Boosting oxygen evolution reaction by activation of lattice-oxygen sites in layered ruddlesden-popper oxide. EcoMat 2020, 2, e12021.
97. Liu, Z.; Li, H.; Kang, H.; N'diaye, A. T.; Lee, M. H. Lattice oxygen-mediated Ni-O-O-M formation for efficient oxygen evolution reaction in MOF@LDH core-shell structures. Chem. Eng. J. 2023, 454, 140403.
98. Liu, C.; Qian, J.; Ye, Y.; et al. Oxygen evolution reaction over catalytic single-site Co in a well-defined brookite TiO2 nanorod surface. Nat. Catal. 2021, 4, 36-45.
99. Jo, S.; Park, W. B.; Lee, K. B.; et al. Bi/BiFe(oxy)hydroxide for sustainable lattice oxygen-boosted electrocatalysis at a practical high current density. Appl. Catal. B:. Environ. 2022, 317, 121685.
100. Xu, J.; Jin, H.; Lu, T.; et al. IrOx·nH2O with lattice water-assisted oxygen exchange for high-performance proton exchange membrane water electrolyzers. Sci. Adv. 2023, 9, eadh1718.
101. Li, Q.; He, Y.; Zhang, L.; et al. Optimizing oxygen transport in proton exchange membrane water electrolysis through tailored porosity configurations of porous transport layers. Appl. Energy. 2024, 370, 123621.
102. Wu, Z. Y.; Chen, F. Y.; Li, B.; et al. Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Nat. Mater. 2023, 22, 100-8.
103. Kong, S.; Li, A.; Long, J.; et al. Acid-stable manganese oxides for proton exchange membrane water electrolysis. Nat. Catal. 2024, 7, 252-61.
104. Zhang, Z.; Jia, C.; Ma, P.; et al. Distance effect of single atoms on stability of cobalt oxide catalysts for acidic oxygen evolution. Nat. Commun. 2024, 15, 1767.
105. Liu, J.; Wang, T.; Lin, Z.; et al. Single-atom Co dispersed on polyoxometalate derivatives confined in bamboo-like carbon nanotubes enabling efficient dual-site lattice oxygen mediated oxygen evolution electrocatalysis for acidic water electrolyzers. Energy. Environ. Sci. 2024, 17, 3088-98.
106. Zhao, H.; Zhu, L.; Yin, J.; et al. Stabilizing lattice oxygen through Mn doping in NiCo2O4-δ spinel electrocatalysts for efficient and durable acid oxygen evolution. Angew. Chem. Int. Ed. Engl. 2024, 63, e202402171.
107. Huo, Y.; Xiu, S.; Meng, L.; Quan, B. Solvothermal synthesis and applications of micro/nano carbons: a review. Chem. Eng. J. 2023, 451, 138572.
108. Jo, S.; Kim, M.; Lee, K. B.; Choi, H.; Zhang, L.; Sohn, J. I. Nonprecious high-entropy chalcogenide glasses-based electrocatalysts for efficient and stable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Adv. Energy. Mater. 2023, 13, 2301420.
109. Thao, N. T. T.; Jang, J. U.; Nayak, A. K.; Han, H. Current trends of iridium-based catalysts for oxygen evolution reaction in acidic water electrolysis. Small. Sci. 2024, 4, 2300109.
110. Li, W.; Bu, Y.; Ge, X.; Li, F.; Han, G. F.; Baek, J. B. Recent advances in iridium-based electrocatalysts for acidic electrolyte oxidation. ChemSusChem 2024, 17, e202400295.
111. Li, W.; Feng, G.; Liu, J.; et al. The structural and chemical reactivity of lattice oxygens on β-PbO2 EOP electrocatalysts. Chinese. J. Struct. Chem. 2022, 41, 2212051-9.
112. Liu, Y.; Liang, X.; Chen, H.; et al. Iridium-containing water-oxidation catalysts in acidic electrolyte. Chinese. J. Catal. 2021, 42, 1054-77.
113. Hu, J.; Jiang, D.; Weng, Z.; et al. A universal electrochemical activation enabling lattice oxygen activation in nickel-based catalyst for efficient water oxidation. Chem. Eng. J. 2022, 430, 132736.
114. Huang, Y.; Pellegrinelli, C.; Wachsman, E. D. Oxygen dissociation kinetics of concurrent heterogeneous reactions on metal oxides. ACS. Catal. 2017, 7, 5766-72.
115. Joshi, B.; Shivashankar, M. Recent advancement in the synthesis of Ir-based complexes. ACS. Omega. 2023, 8, 43408-32.
116. Chen, L.; Liang, H. Ir-based bifunctional electrocatalysts for overall water splitting. Catal. Sci. Technol. 2021, 11, 4673-89.
117. Xie, Y.; Yu, X.; Li, X.; Long, X.; Chang, C.; Yang, Z. Stable and high-performance Ir electrocatalyst with boosted utilization efficiency in acidic overall water splitting. Chem. Eng. J. 2021, 424, 130337.
118. Xie, Y.; Long, X.; Li, X.; Chang, C.; Qu, K.; Yang, Z. The template synthesis of ultrathin metallic Ir nanosheets as a robust electrocatalyst for acidic water splitting. Chem. Commun. 2021, 57, 8620-3.
119. Kwon, J.; Sun, S.; Choi, S.; et al. Tailored electronic structure of Ir in high entropy alloy for highly active and durable bifunctional electrocatalyst for water splitting under an acidic environment. Adv. Mater. 2023, 35, e2300091.
120. Yang, M.; Zhou, K.; Wang, C.; et al. Iridium single-atom catalyst coupled with lattice oxygen activated CoNiO2 for accelerating the oxygen evolution reaction. J. Mater. Chem. A. 2022, 10, 25692-700.
121. Wu, J.; Zou, W.; Zhang, J.; et al. Regulating Ir-O covalency to boost acidic oxygen evolution reaction. Small 2024, 20, e2308419.
122. Wang, Y.; Liu, S.; Qin, Q.; et al. Praseodymium iridium oxide as a competitive electrocatalyst for oxygen evolution reaction in acid media. Sci. China. Mater. 2021, 64, 2193-201.
123. Shi, Z.; Wang, Y.; Li, J.; et al. Confined Ir single sites with triggered lattice oxygen redox: toward boosted and sustained water oxidation catalysis. Joule 2021, 5, 2164-76.
124. Zhu, W.; Song, X.; Liao, F.; et al. Stable and oxidative charged Ru enhance the acidic oxygen evolution reaction activity in two-dimensional ruthenium-iridium oxide. Nat. Commun. 2023, 14, 5365.
125. Long, X.; Zhao, B.; Zhao, Q.; et al. Ru-RuO2 nano-heterostructures stabilized by the sacrificing oxidation strategy of Mn3O4 substrate for boosting acidic oxygen evolution reaction. Appl. Catal. B:. Environ. 2024, 343, 123559.
126. Vijayakumar, P.; Lenus, S.; Pradeeswari, K.; et al. In situ reconstructed layered double hydroxides via MOF engineering and Ru doping for decoupled acidic water oxidation enhancement. Energy. Fuels. 2024, 38, 4504-15.
127. Jin, H.; Liu, X.; An, P.; et al. Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 2023, 14, 354.
128. Zhou, G.; Wang, P.; Hu, B.; et al. Spin-related symmetry breaking induced by half-disordered hybridization in BixEr2-xRu2O7 pyrochlores for acidic oxygen evolution. Nat. Commun. 2022, 13, 4106.
129. Wen, Y.; Liu, C.; Huang, R.; et al. Introducing brønsted acid sites to accelerate the bridging-oxygen-assisted deprotonation in acidic water oxidation. Nat. Commun. 2022, 13, 4871.
130. Wang, H.; Yan, Z.; Cheng, F.; Chen, J. Advances in noble metal electrocatalysts for acidic oxygen evolution reaction: construction of under-coordinated active sites. Adv. Sci. 2024, 11, e2401652.
131. Chang, J.; Shi, Y.; Wu, H.; et al. Oxygen radical coupling on short-range ordered Ru atom arrays enables exceptional activity and stability for acidic water oxidation. J. Am. Chem. Soc. 2024, 146, 12958-68.
132. Wang, K.; Wang, Y.; Yang, B.; et al. Highly active ruthenium sites stabilized by modulating electron-feeding for sustainable acidic oxygen-evolution electrocatalysis. Energy. Environ. Sci. 2022, 15, 2356-65.
133. Zhu, W.; Yao, F.; Cheng, K.; et al. Direct dioxygen radical coupling driven by octahedral ruthenium-oxygen-cobalt collaborative coordination for acidic oxygen evolution reaction. J. Am. Chem. Soc. 2023, 145, 17995-8006.
134. He, J.; Chen, W.; Gao, H.; et al. Tuning hydrogen binding modes within RuO2 lattice by proton and electron co-doping for active and stable acidic oxygen evolution. Chem. Catal. 2022, 2, 578-94.
135. Li, X.; Li, C.; Xie, Y.; Pan, S.; Luo, F.; Yang, Z. Anion effect on oxygen reduction reaction activity of nitrogen doped carbon nanotube encapsulated cobalt nanoparticles. Appl. Surf. Sci. 2024, 648, 158975.
136. Luo, F.; Pan, S.; Xie, Y.; Li, C.; Yu, Y.; Yang, Z. Atomically dispersed Ni electrocatalyst for superior urea-assisted water splitting. J. Energy. Chem. 2024, 90, 1-6.
137. Cui, X.; Ren, P.; Deng, D.; Deng, J.; Bao, X. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy. Environ. Sci. 2016, 9, 123-9.
138. Liu, J.; Ning, G.; Shi, K.; et al. N-doped hollow porous carbon spheres@Co Cu Fe alloy nanospheres as novel non-precious metal electrocatalysts for HER and OER. Inter. J. Hydrogen. Energy. 2022, 47, 5947-60.
139. Tan, B.; Huo, Z.; Sun, L.; et al. Ionic liquid-modulated synthesis of MnO2 nanowires for promoting propane combustion: microstructure engineering and regulation mechanism. Colloids. . Surf. A:. Phy. Eng. Aspects. 2023, 656, 130431.
140. Selvadurai, A. PB, Xiong T, Huang P, et al. Tailoring the cationic and anionic sites of LaFeO3 -based perovskite generates multiple vacancies for efficient water oxidation. J. Mater. Chem. A. 2021, 9, 16906-16.
141. Shi, Y.; Ma, Z. R.; Xiao, Y. Y.; et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021.
142. Ham, K.; Kang, S.; Kim, Y.; Lee, Y.; Kim, Y.; Lee, J. Participation of the unstable lattice oxygen of cation-exchanged δ-MnO2 in the water oxidation reaction. J. Mater. Chem. A. 2023, 11, 21686-93.
143. Shi, Z.; Li, J.; Jiang, J.; et al. Enhanced acidic water oxidation by dynamic migration of oxygen species at the Ir/Nb2O5-x catalyst/support interfaces. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212341.
144. Yao, Y.; Hu, S.; Chen, W.; et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. Nat. Catal. 2019, 2, 304-13.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.











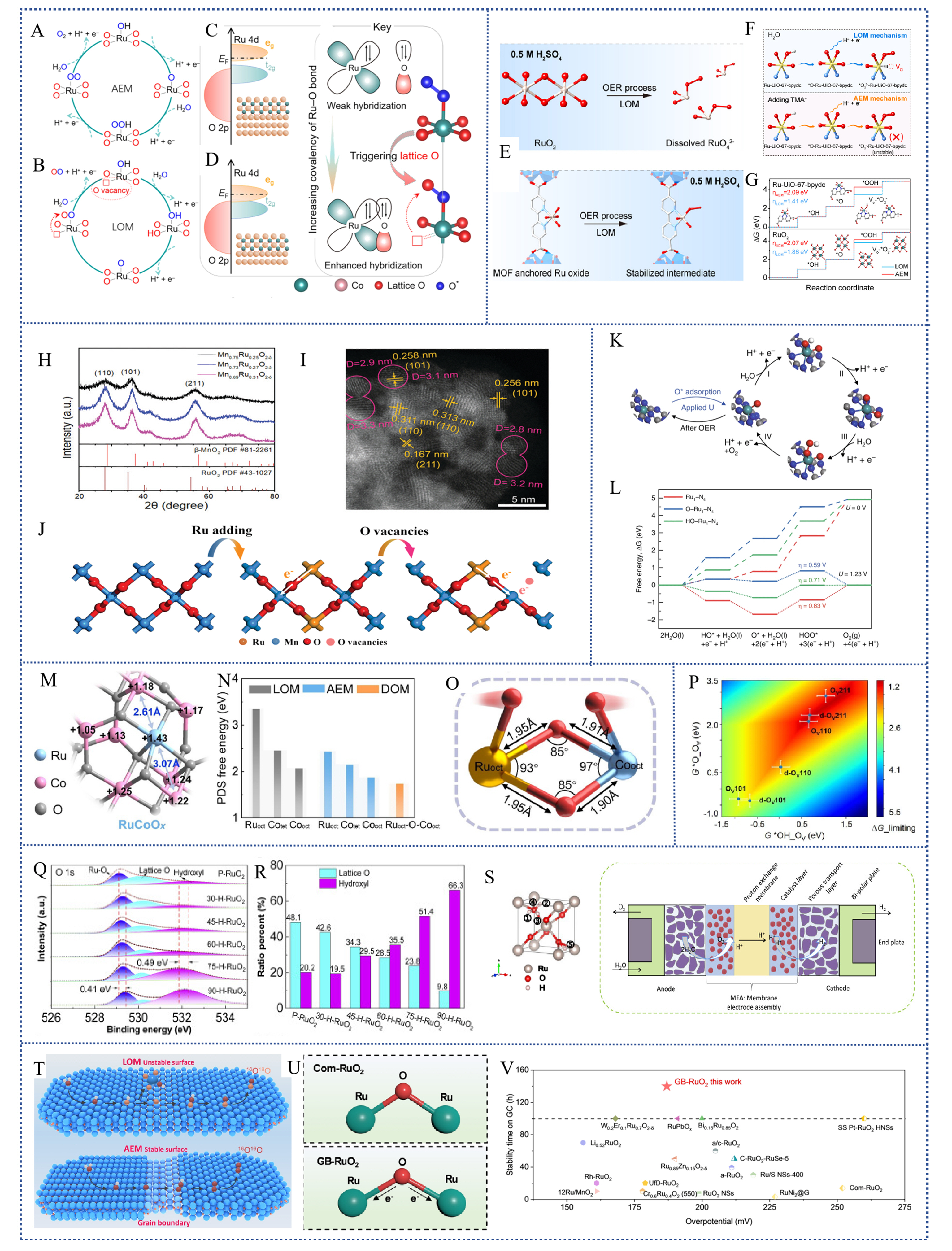















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].