fig7
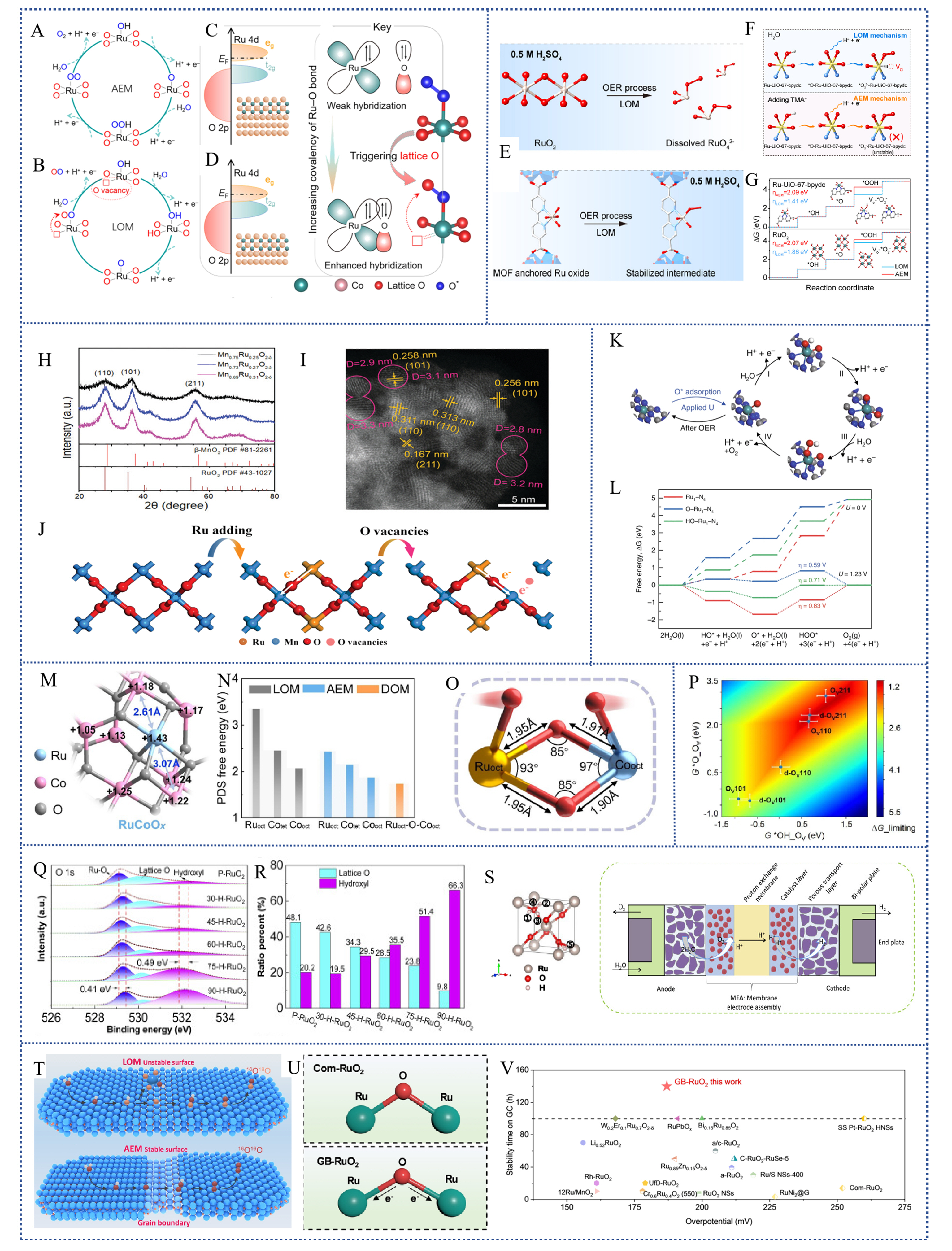
Figure 7. (A) AEM and (B) LOM pathways of Ru-based electrocatalysts; (C) Overlap between Ru 4d and the O 2p ligand state during AEM and (D) orbital optimization leading to the LOM: Ru 4d center is downshifted to penetrate the O 2p ligand band, leading to increased filling of the antibonding (eg) state, and modulation of orbital hybridization for increasing the Ru-O covalency, thus activating the participation of lattice O in the OER. (Copyright 2023, American Chemical Society[131]); (E) Schematic representation of the oxidation of RuO2 to a high valence state in the acidic OER of the LOM pathway (upper panel) and the stability of the MOF-anchored Ru intermediate (lower panel); (F) Schematic representation of CV peak intermediates with and without TMA+ and (J) Gibbs free energy step diagrams for Ru-UiO-67-bpydc and RuO2 catalysts during OER via AEM or LOM pathway (Copyright 2023, Elsevier); XRD diagram (H) and HAADF-STEM image (I) of Mn0.73Ru0.27O2-δ and the charge redistribution process (J) in the oxide after the addition of Ru (Copyright 2022, Royal Society of Chemistry); (K) Free energy diagram for OER on Ru1-N4, O-Ru1-N4, and HO-Ru1-N4; (L) Schematic of the whole OER mechanism on Ru-N-C in the acidic electrolyte (Copyright 2019, Springer Nature); (M) Bader charge distribution diagram of RuCoOx and (N) comparison of PDS energy barriers for different reaction pathways; (O) Model of the Ruoct-O-Cooct structure with two adsorbed oxygen radicals (Copyright 2023, American Chemical Society[133]); (P) A two-dimensional activity map for LOM-OVSM mechanism (Copyright 2023, Springer Nature[95]); (Q) O 1s fitting plots for different H-doped RuO2 and (R) the ratio of V-O to O-H in the O 1s fitting results for a series of H-RuO2; (S) Structural maps of the five sites of H in RuO2 and schematic diagrams using the SPE device (Copyright 2022, Elsevier[134]); (T) Schematic structure of LOM converted to AEM in rich grain boundaries; (U) Ru-O elongation as well as electronic shift in GB-RuO2; (V) Comparison of acidic OER stability and activity of RuO2-based catalysts (Copyright 2024, John Wiley and Sons[93]). AEM: Adsorption evolution mechanism; LOM: Lattice oxygen mechanism; OER: Oxygen generation reaction; TMA: TetraMethylAmmonium; MOF: Metal organic framework; XRD: X-ray diffraction; PDS: Potential-determining step; LOM-OVSM: Lattice oxygen mechanism-oxygen vacancy site mechanism.









