Intraoperative celiac axis flow measurement and dynamic surgical strategies in robotic distal pancreatectomy
Abstract
Locally advanced pancreatic ductal adenocarcinoma (LA-PDAC) involving the body and neck of the pancreas often necessitates complex resections in selected cases, such as the Appleby procedure, also known as distal pancreatectomy with en bloc celiac axis resection (DP-CAR). A 75-year-old female with LA-PDAC, confirmed by endoscopic ultrasound-guided biopsy and treated with neoadjuvant chemotherapy, was initially planned for a robotic DP-CAR. During surgery, transit-time flow measurement (TTFM) showed an inverted and threefold increase in the gastroduodenal artery flow after clamping of the common hepatic artery (CHA), ensuring adequate liver perfusion and avoiding unnecessary preoperative CHA embolization to enhance collateral arterial circulation to the liver. Intraoperative findings revealed only fibrosis without evidence of tumor infiltration around the celiac axis, as confirmed by intraoperative frozen sections. This led to a shift from the planned DP-CAR procedure to an anterior radical antegrade modular pancreatosplenectomy (RAMPS). Final histopathological examinations revealed chronic pancreatitis and complete tumor regression (tumor regression grade 0, ypT0N0). This case underscores the role of robotic platforms and intraoperative tools like TTFM in real-time decision making, enabling tailored surgical strategies for complex pancreatic resections, ensuring oncological adequacy, and optimizing patient outcomes.
Keywords
INTRODUCTION
Locally advanced pancreatic ductal adenocarcinoma (LA-PDAC) involving the body and neck of the pancreas poses significant surgical challenges due to its close proximity to critical vascular structures such as the celiac axis (CA). Originally proposed by Appleby in 1953 for locally advanced gastric cancer with CA involvement[1], the technique of CA resection was later adapted for pancreatic surgery as the modified Appleby procedure or distal pancreatectomy with CA resection (DP-CAR)[2], becoming a pivotal strategy in order to achieve negative margins in LA-PDAC.
This adaptation has facilitated more aggressive resections in selected patients, improving oncologic outcomes while addressing vascular involvement[3,4].
The rationale for performing CA resection without causing arterial hepatic ischemia is based on the presence of a patent collateral arterial network, the pancreaticoduodenal arcade (Rio Branco’s arcade). This arcade, formed by the anastomosis of the superior pancreaticoduodenal artery [originating from the gastroduodenal artery (GDA)] and the inferior pancreaticoduodenal artery [arising from the superior mesenteric artery (SMA)], allows for retrograde liver perfusion via the SMA in case of common hepatic artery (CHA) occlusion or ligation[5]. This vascular configuration provides the hemodynamic basis for safely performing DP-CAR while preserving hepatic arterial flow.
Traditionally, this procedure relies on an initial stage of CHA ligation or embolization to ensure adequate adaptation of collateral arterial flow[6]. However, advances in intraoperative technologies, such as transit-time flow measurement (TTFM), now enable real-time assessment of arterial flow, providing a precise and reproducible evaluation of collateral pathways such as the GDA[7,8]. This technique offers a precious tool to confirm hemodynamic adequacy directly during surgery.
The advent of robotic surgery has further refined pancreatic procedures by enhancing precision and reducing invasiveness. In particular, robotic-assisted DP-CAR has shown promising results in terms of feasibility, safety, and oncologic outcomes, particularly when performed in high-volume centers[9]. Indeed, the robotic-assisted approach significantly reduces operative time, minimizes blood loss, and lowers transfusion rates compared to open surgery[10]. Such benefits highlight its value in carefully selected patients undergoing multimodal treatment, underscoring the role of robotic surgery in advancing pancreatic cancer care[11].
In the presented case, a robotic-assisted approach was employed, with the intraoperative use of TTFM probes to measure flow gradients in the GDA as part of the planned DP-CAR, which was indicated due to preoperative imaging findings of celiac axis encasement. However, intraoperative findings revealed that the presumed neoplastic infiltration of the CA observed on preoperative imaging was, in fact, fibrotic tissue likely resulting from chemotherapy. This pivotal discovery led to a strategic shift, transitioning from the planned robotic-assisted DP-CAR to an alternative yet equally demanding procedure: a robotic anterior radical antegrade modular pancreatosplenectomy (RAMPS)[12].
The Supplementary Video of the robotic-assisted procedure is included, showcasing the technical execution of flow gradient measurement.
CASE PRESENTATION AND SURGICAL TECHNIQUE
A 75-year-old female patient with a BMI of 29.1 kg/m2 presented for surgical management of LA-PDAC involving the body and isthmus of the pancreas. Her medical history included hypertension, hypertensive cardiomyopathy, chronic obstructive pulmonary disease, gastroesophageal reflux disease, hiatal hernia, and chronic gastritis. She denied prior abdominal surgeries and had no known allergies.
At the time of diagnosis, a contrast-enhanced CT scan revealed a pancreatic lesion measuring 42 × 26 mm with encasement of the CA. This finding was subsequently confirmed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), which established the diagnosis of PDAC. EUS findings also corroborated the involvement of the CA. CA19-9 levels were elevated (400 U/mL). Based on these findings, the multidisciplinary team (MDT) recommended conversion chemotherapy with the goal of achieving surgical resectability. Thus, the patient underwent six cycles of mFOLFIRINOX, resulting in CA19-9 normalization to 4.6 U/mL and tumor size reduction, with restaging CT, magnetic resonance imaging (MRI), and positron emission tomography (PET) revealing a hypodense 25 × 14 mm pancreatic lesion, persistent CA encasement, but overall decreased dimensions [Figure 1].
The MDT reviewed the case and recommended surgical resection, with an initial plan for a DP-CAR. Preoperative assessments indicated good functional status with an ASA score of 2, a Karnofsky score of 90, and ECOG 0.
The patient was admitted and underwent robotic-assisted surgery during the same hospitalization.
The operation began with open periumbilical access, followed by staging laparoscopy, which excluded peritoneal or liver metastases. Four robotic ports and an assistant 12 mm laparoscopic port were placed, and the Da Vinci Xi (Intuitive Surgical Inc.) system was docked.
The gastrocolic ligament was incised to access the omental bursa and expose the pancreas. The right gastroepiploic vessels were ligated and divided, and the stomach was suspended with a transabdominal stitch to facilitate further dissection.
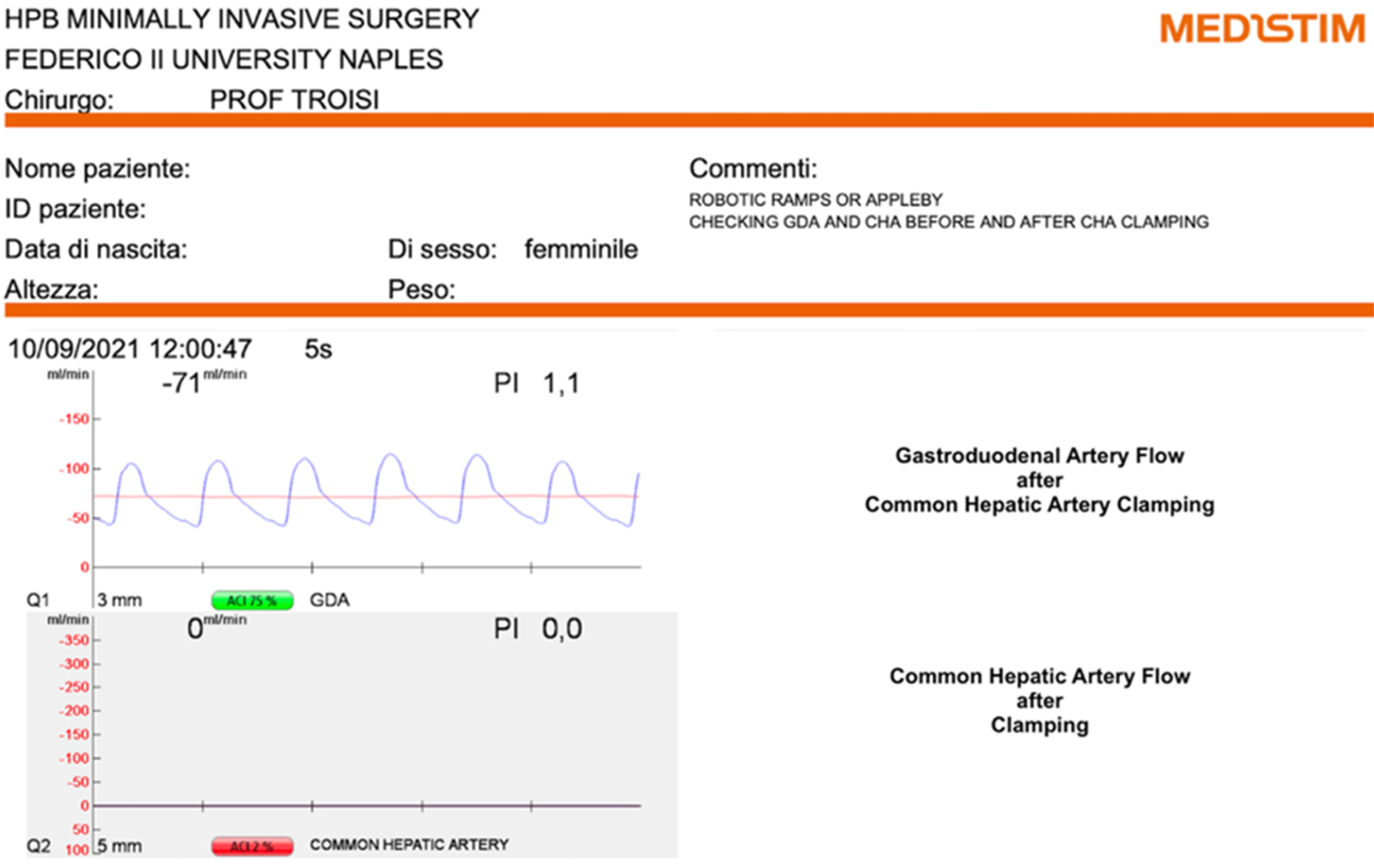
The CA was carefully dissected and exposed. TTFM (Medistim) was employed to assess arterial flow dynamics [Figure 2]. Before clamping the CHA, TTFM recorded a flow of 24 mL/min [pulsatility index (PI) 1.7] in the GDA [Figure 3]. After CHA clamping, the GDA flow was inverted and increased to 71 mL/min (PI 1.1), representing an approximately 3-fold increase in absolute flow [Figure 4]. This result demonstrated that functional arterial flow could be achieved without preoperative embolization of the CHA.
Figure 2. TTFM probes positioned around CHA and GDA. TTFM: Transit-time flow measurement; CHA: common hepatic artery; GDA: gastroduodenal artery.
Figure 3. Assessment of basal blood flow in the GDA and CHA using TTFM probes. GDA: Gastroduodenal artery; CHA: common hepatic artery; TTFM: transit-time flow measurement.
Figure 4. Assessment of basal blood flow in the GDA and CHA using TTFM probes after clamping of the CHA. GDA: Gastroduodenal artery; CHA: common hepatic artery; TTFM: transit-time flow measurement.
The lymphadenectomy around the CA was then performed. Fibrotic tissue surrounding the CA was identified, and the absence of macroscopic evidence of malignancy was confirmed by intraoperative frozen sections. Intraoperative ultrasound (IOUS) was used to precisely localize the lesion and define its anatomical relationships.
Based on the absence of vascular infiltration around the CA, confirmed by negative intraoperative frozen section, the planned robotic DP-CAR procedure was revised to a robotic anterior RAMPS.
The splenic artery was isolated and divided using Hem-o-lok clips. The splenic vein was divided concurrently with the transection of the pancreatic parenchyma using a robotic stapler to the left of the superior mesenteric vein. The pancreatic body and tail were carefully mobilized with the preservation of the left adrenal gland. The retroperitoneal areolar tissue was also dissected, with the dissection plane lying directly anterior to the surface of the left adrenal gland and Gerota’s fascia. The procedure was completed with the lysis of the splenic fixation structures.
Hemostasis was ensured, and a CH 24 abdominal drain was placed in the splenopancreatic bed. Blood loss was estimated at 350 mL, and the procedure lasted 320 min.
The Supplementary Video clip accompanying this case demonstrates the surgical steps and the critical intraoperative decision-making process.
The postoperative course was uneventful. The patient resumed oral intake on postoperative day (POD) 2, with minimal drain output and no complications such as pancreatic fistula or hemorrhage. She was discharged on POD 10 in good condition and tolerating a regular diet.
Histopathological analysis showed a complete regression of the disease, with findings consistent with chronic pancreatitis featuring extensive fibrosis and acinar atrophy. These changes were interpreted as likely related to post-chemotherapy effects. Tumor regression grade (TRG) 0 was assigned, and the pathological staging was classified as ypT0N0. This outcome corroborated the intraoperative observations and justified the surgical strategy adjustment to an anterior RAMPS.
During follow-up, the patient underwent CT and MRI examinations according to the scheduled surveillance plan. Three years after surgery, MRI revealed a pancreatic head lesion, raising suspicion of disease progression despite the initial complete pathological response.
DISCUSSION
This case demonstrates the critical role of intraoperative assessment in guiding the surgical decision-making process during complex pancreatic resections. Robotic systems offer significant advantages in complex hepatopancreatobiliary surgeries, providing a shorter learning curve and facilitating the reproducibility of intricate procedures, as highlighted in recent literature on the application of robotic platforms in hepatobiliopancreatic surgery[13]. The planned robotic DP-CAR procedure was revised intraoperatively to an anterior RAMPS based on the absence of CA infiltration. This highlights the adaptability of robotic platforms like the Da Vinci Xi (Intuitive Surgical Inc.), which enable precise real-time evaluation and surgical strategy adjustments, even in the context of inherently technically demanding procedures further complicated by fibrotic outcomes from preoperative chemotherapy.
One of the key aspects of this case was the use of TTFM (Medistim) to assess arterial hemodynamics in the GDA in preparation for the planned DP-CAR procedure. Before clamping the CHA, the GDA flow was measured. After CHA clamping, the GDA flow inverted and increased by approximately threefold. These findings provided critical confirmation of sufficient collateral perfusion through the GDA, making preoperative embolization of the CHA unnecessary. This approach reduces procedural complexity, shortens the overall treatment timeline, and minimizes the risks associated with preoperative embolization, such as liver ischemia or delayed surgery due to insufficient collateralization.
The MDT discussion was crucial in tailoring treatment, ensuring an oncologically sound and adaptable surgical strategy based on preoperative chemotherapy response and intraoperative findings. In this regard, recent literature highlights the effectiveness of MDT approaches, particularly in borderline resectable and locally advanced pancreatic cancers, in optimizing treatment strategies, improving patient selection for surgery, and enhancing overall outcomes[14].
The histopathological findings of chronic pancreatitis with extensive fibrosis further emphasized the importance of intraoperative decision making. While preoperative imaging suggested potential neoplastic involvement of the CA, the intraoperative findings showed fibrotic changes likely induced by preoperative chemotherapy. These post-treatment effects highlight the challenges of accurately assessing vascular involvement based solely on preoperative imaging, underscoring the need for real-time surgical evaluation.
A comparable impact of preoperative treatments has been observed in other malignancies. Grosso et al. reported cases of metastatic renal cell carcinoma where systemic treatment with pembrolizumab and axitinib led to significant tumor regression, facilitating a more conservative surgical approach[15]. This underscores the transformative potential of preoperative therapies in complex oncologic cases.
Building upon this concept, the integration of advanced preoperative planning tools, such as three-dimensional virtual models, has further enhanced the precision of surgical interventions. These tools not only improve anatomical visualization and facilitate preoperative planning but also complement intraoperative technologies like TTFM, contributing to a more refined and data-driven surgical approach. By reducing intraoperative uncertainties and optimizing resection strategies, they support better functional outcomes, particularly in high-risk cases[16].
TTFM has been highlighted as a valuable tool for intraoperative flow assessment, offering continuous and quantitative volumetric measurements. Compared to Doppler ultrasound, which is angle-dependent and subject to interobserver variability, TTFM provides more reliable data, making it particularly suitable for intraoperative vascular assessment. Studies have demonstrated its advantages in liver transplantation, where it enables precise hemodynamic monitoring and correlates with graft survival and functional outcomes[8,17,18]. While Doppler ultrasound remains useful for preoperative and postoperative evaluations, its intraoperative limitations make TTFM a preferred choice in intraoperative arterial flow assessment[8].
In our case, collateral flow through the GDA reached 71 mL/min after CHA clamping, which was deemed sufficient based on intraoperative assessment and systemic hemodynamic stability. While arterial flows below 100 mL/min have been associated with a higher risk of ischemic complications in liver transplantation[17], no universal threshold is established for other hepatobiliary and pancreatic procedures. Instead of relying solely on a percentage increase after CHA clamping, intraoperative evaluation should consider absolute flow values, systemic hemodynamics, and collateral recruitment[8]. These parameters, assessed using direct measurement tools like TTFM, provide a more comprehensive evaluation of hepatic arterial perfusion[8].
In cases where GDA blood flow is deemed insufficient after CHA clamping during a planned DP-CAR, several bailout strategies can be considered. One option is intraoperative arterial revascularization, including anastomosis of the splenic artery to the hepatic artery, which has been described as a feasible method to restore hepatic perfusion[19]. Alternatively, interposition grafts, such as reversed saphenous vein grafts between the CA stump and the CHA, can augment hepatic and gastric blood flow, potentially preventing complications such as hepatic abscess or delayed gastric emptying[20]. Another approach is preoperative embolization of the CHA or CA to stimulate the development of collateral circulation, thereby improving hepatic perfusion during surgery. This strategy aims to promote increased arterial collateral blood flow in preexisting pathways, facilitating safer resection without the need for complex arterial reconstructions[6,21]. On the other hand, in cases of locally advanced tumors deemed unresectable, non-surgical alternatives such as electrochemotherapy have been explored to enhance tumor control and provide a palliative or cytoreductive approach[22].
Assessing vascular invasion is crucial in surgical planning, especially post-conversion therapy, where fibrosis can mimic tumor invasion. To improve preoperative assessment, PET-CT after chemotherapy has been shown to aid in distinguishing viable tumors from fibrosis, refining surgical planning and decision making[23]. Additionally, a recent study demonstrated that endoscopic ultrasound elastography (EUS-EG) enhances diagnostic accuracy over standard EUS and CT, with higher sensitivity (91.7%) and specificity (90.0%) compared to dynamic CT (73.3% and 69.7%, respectively)[24]. However, intraoperative findings may still reveal fibrosis that appears similar to tumor infiltration, increasing the risk of violating oncologic principles. To mitigate this, intraoperative frozen section analysis[25] and IOUS[26] have been shown to aid in confirming vascular status.
This case also exemplifies the advantages of robotic-assisted surgery in managing pancreatic tumors. Robotic platforms provide enhanced visualization, precision, and dexterity, which are essential for dissecting complex vascular structures and achieving negative margins[27]. The ability to integrate advanced technologies like TTFM into robotic surgery workflows further improves surgical outcomes by enabling personalized and data-driven decisions during the procedure.
From an oncological perspective, the decision to perform an anterior RAMPS ensured oncological adequacy while avoiding the additional risks and morbidity associated with the more extensive Appleby procedure. The anterior RAMPS approach effectively addressed the patient’s tumor burden while preserving vital vascular structures, contributing to a smooth postoperative recovery and favorable short-term outcomes.
CONCLUSIONS
This case highlights the synergistic benefits of robotic platforms and advanced intraoperative technologies, such as TTFM, in pancreatic surgery. The integration of precise imaging, hemodynamic assessment, and surgical adaptability allows for tailored strategies that optimize oncological and functional outcomes.
DECLARATIONS
Authors’ contributions
Conceived and designed the case report, and wrote the manuscript: Loiaco G
Supervised the preparation of the case report, providing critical feedback and guidance: Rompianesi G
Assisted in intraoperative procedures and surgical support: Campanile S
Contributed to the scientific analysis and interpretation of the case: Giglio M
Collected and organized the clinical data relevant to the case: Caggiano M, Pacilio B
Contributed scientific expertise, offering critical insights and fostering academic rigor: Montalti R
Oversaw the project as head of the department, offering strategic direction and final approval for the publication: Troisi RI
Availability of data and materials
All relevant clinical data supporting this case report are presented within the article. However, the complete clinical documentation cannot be shared publicly to ensure patient confidentiality and privacy.
Financial support and sponsorship
None.
Conflicts of interest
Montalti R is an Editorial Board member of the journal Mini-invasive Surgery. Montalti R was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, and decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The case report complies with the Declaration of Helsinki. Written informed consent was obtained from the patient for the publication of this case report. Ethical approval was not required as this is a single case report.
Consent for publication
Written informed consent was obtained from the patient for the publication of their medical records and any accompanying images. All procedures were conducted in accordance with ethical guidelines and institutional regulations.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer. 1953;6:704-7.
2. Sindayigaya R, Barat M, Tzedakis S, et al. Modified Appleby procedure for locally advanced pancreatic carcinoma: a primer for the radiologist. Diagn Interv Imaging. 2023;104:455-64.
3. Egorov V, Kim P, Kharazov A, et al. Hemodynamic, surgical and oncological outcomes of 40 distal pancreatectomies with celiac and left gastric arteries resection (DP CAR) without arterial reconstructions and preoperative embolization. Cancers. 2022;14:1254.
4. Klompmaker S, Peters NA, van Hilst J, et al; E-AHPBA DP-CAR study group. Outcomes and risk score for distal pancreatectomy with celiac axis resection (DP-CAR): an international multicenter analysis. Ann Surg Oncol. 2019;26:772-81.
5. Favelier S, Germain T, Genson PY, et al. Anatomy of liver arteries for interventional radiology. Diagn Interv Imaging. 2015;96:537-46.
6. Cannella R, Borhani AA, Zureikat AH, Tublin ME. Appleby procedure (distal pancreatectomy with celiac artery resection) for locally advanced pancreatic carcinoma: indications, outcomes, and imaging. AJR Am J Roentgenol. 2019;213:35-44.
7. Mittal A, de Reuver PR, Shanbhag S, et al. Distal pancreatectomy, splenectomy, and celiac axis resection (DPS-CAR): common hepatic arterial stump pressure should determine the need for arterial reconstruction. Surgery. 2015;157:811-7.
8. Bassas X, Hente E. Different methods for hepatic flow measurements: a narrative review. Acta Anaest Belg. 2023;74:289-96.
9. Greer J, Zureikat AH. Robotic distal pancreatectomy combined with celiac axis resection. J Vis Surg. 2017;3:145.
10. Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB. 2016;18:835-42.
11. Beane JD, Zureikat AH. Robotic distal pancreatectomy with celiac axis resection for locally advanced pancreatic cancer. Ann Pancreat Cancer. 2018;1:11-11.
12. Kitagawa H, Tajima H, Nakagawara H, et al. A modification of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the left pancreas: significance of en bloc resection including the anterior renal fascia. World J Surg. 2014;38:2448-54.
13. Troisi RI, Rompianesi G, Giglio MC, Montalti R. The democratizing effects of robotic surgery: nine HPB manoeuvres exactly reproduced by the da vinci system. Surg Oncol. 2022;44:101822.
14. Yao W, Chen X, Fan B, et al. Multidisciplinary team diagnosis and treatment of pancreatic cancer: current landscape and future prospects. Front Oncol. 2023;13:1077605.
15. Grosso AA, DI Maida F, Mari A, Raspollini MR, Antonuzzo L, Minervini A. Complete response of metastatic RCC with caval vein thrombus following treatment with pembrolizumab and axitinib: is it possible to extend the indications for systemic therapy? Minerva Urol Nephrol. 2024;76:382-4.
16. Grosso AA, Di Maida F, Lambertini L, et al. Three-dimensional virtual model for robot-assisted partial nephrectomy: a propensity-score matching analysis with a contemporary control group. World J Urol. 2024;42:338.
17. Sainz-Barriga M, Reyntjens K, Costa MG, et al. Prospective evaluation of intraoperative hemodynamics in liver transplantation with whole, partial and DCD grafts. Am J Transplant. 2010;10:1850-60.
18. Pratschke S, Meimarakis G, Mayr S, et al. Arterial blood flow predicts graft survival in liver transplant patients. Liver Transpl. 2011;17:436-45.
19. de Santibañes M, Alvarez FA, Mazza OM, et al. Surgical strategies for restoring liver arterial perfusion in pancreatic resections. Langenbecks Arch Surg. 2016;401:113-20.
20. Christians KK, Pilgrim CH, Tsai S, et al. Arterial resection at the time of pancreatectomy for cancer. Surgery. 2014;155:919-26.
21. Storkholm JH, Burgdorf SK, Larsen PN, Hansen CP. Pancreaticoduodenectomy with preoperative total embolization of the hepatic arteries (PD-HAE)-a novel treatment with sacrifice of the hepatic arterial blood supply without the need for arterial reconstruction. Langenbecks Arch Surg. 2023;408:310.
22. Rompianesi G, Loiaco G, Rescigno L, et al. A systematic review of indications and clinical outcomes of electrochemotherapy in pancreatic ductal adenocarcinoma. Cancers. 2025;17:408.
23. Evangelista L, Zucchetta P, Moletta L, et al. The role of FDG PET/CT or PET/MRI in assessing response to neoadjuvant therapy for patients with borderline or resectable pancreatic cancer: a systematic literature review. Ann Nucl Med. 2021;35:767-76.
24. Yamada K, Kawashima H, Ohno E, et al. Diagnosis of vascular invasion in pancreatic ductal adenocarcinoma using endoscopic ultrasound elastography. BMC Gastroenterol. 2020;20:81.
25. Dikmen K, Kerem M, Bostanci H, Sare M, Ekinci O. Intra-operative frozen section histology of the pancreatic resection margins and clinical outcome of patients with adenocarcinoma of the head of the pancreas undergoing pancreaticoduodenectomy. Med Sci Monit. 2018;24:4905-13.
26. Michiels N, Doppenberg D, Groen JV, et al; Dutch Pancreatic Cancer Group. Intraoperative ultrasound during surgical exploration in patients with pancreatic cancer and vascular involvement (ULTRAPANC): a prospective multicenter study. Ann Surg Oncol. 2023;30:3455-63.
Cite This Article
How to Cite
Loiaco, G.; Rompianesi, G.; Giglio, M.; Campanile, S.; Caggiano, M.; Pacilio, B.; Montalti, R.; Troisi, R. I. Intraoperative celiac axis flow measurement and dynamic surgical strategies in robotic distal pancreatectomy. Mini-invasive. Surg. 2025, 9, 9. http://dx.doi.org/10.20517/2574-1225.2024.116
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data





















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].