fig2
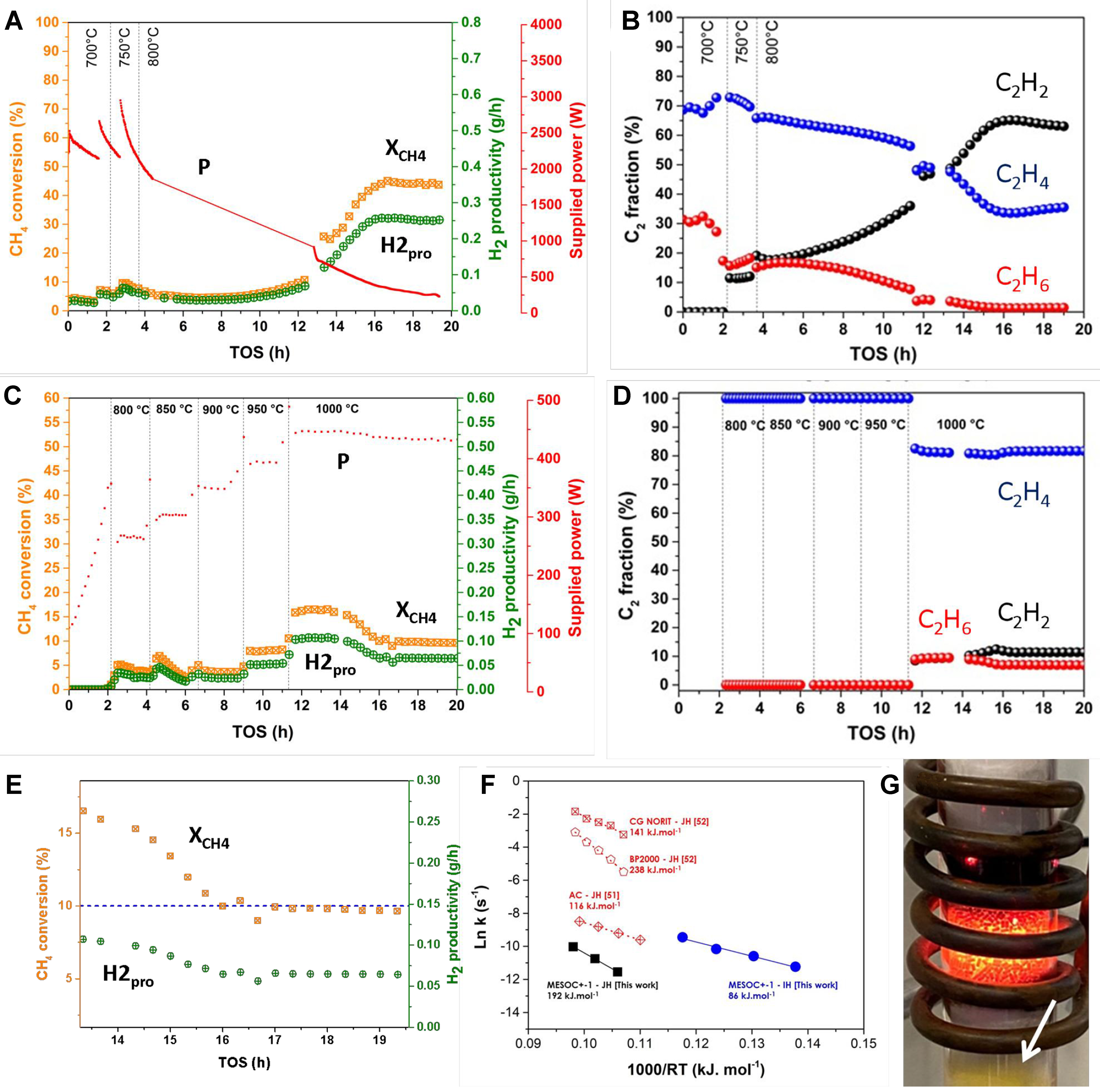
Figure 2. (A) CH4 conversion (XCH4), H2 productivity (H2pro), supplied power (P) and (B) C2H2, C2H4 and C2H6 selectivity as a function of reaction temperature and TOS on the MESOC+-1 (R0) catalyst as a function of temperature and TOS under direct IH; (C) CH4 conversion (XCH4), H2 productivity (H2pro), supplied power (P) and (D) C2H2, C2H4 and C2H6 selectivity on the same carbon catalyst as a function of temperature and TOS under indirect JH. The methane flow rate is set at 60 mL/min corresponding to a WHSV of








