Pneumonia promotes pulmonary metastasis of HCC after transplantation via VEGF regulated PI3K/AKT/Cas-9 signaling and angiogenesis
Abstract
Aim: Pneumonia is the most frequent early postoperative complication in liver transplantation (LT) recipients. Inflammation may provide a favorable environment for tumor implantation, so we aimed to evaluate the impact of pneumonia on pulmonary metastasis of hepatocellular carcinoma (HCC) and reveal its underlying mechanism.
Methods: A training cohort with 234 LT recipients were recorded and analyzed. Using the propensity-score method, we matched covariates between patients with and without pneumonia. A model for predicting pulmonary metastasis was built and validated in an independent validating cohort containing 179 subjects. A mouse model was built to mimic HCC pulmonary metastasis. The potential pathway was revealed by cytokine array analysis and validated in vitro.
Results: Pneumonia was an independent risk factor for pulmonary metastasis in liver transplant recipients. It promoted pulmonary metastasis in both the clinical setting and the mouse model. In vitro, LPS-stimulated VEGF secretion from macrophages in the lung significantly reduced cell apoptosis and activated PI3K/AKT/cas-9 signaling. Administration of VEGF receptor2 inhibitor Vatalanib could reduce metastasis and improve prognosis in pneumonia mice.
Conclusion: Pneumonia promotes HCC pulmonary metastasis by activating PI3K/AKT/Cas-9 signaling in HCC cells via macrophage-originated VEGF. Vatalanib might be efficient in reducing HCC pulmonary metastasis in liver transplant recipients with pneumonia.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common lethal cancer and ranks third among cancer-related deaths worldwide[1]. A net increase of 62% in HCC-related annual death rate was seen in the past two decades[2]. Liver transplantation (LT) provides a possible cure for both HCC and underlying liver disease[3]. However, HCC metastasis is a major obstacle to long-term survival after LT. Although candidate selection criteria such as Milan Criteria[4] were introduced, and various strategies such as donor liver preservation, down-staging and targeted therapy were recently developed for reducing the recurrence risk, the 5-year recurrence rates were not dramatically improved, ranging from 10.0% to 23.6%[5-7]. Looking deeper into the recurrence/metastasis type, we know that typically, HCC recurrence occurs intrahepatically after partial hepatic resection. In contrast, extrahepatic metastasis, particularly pulmonary metastasis, is the major pattern after LT. The incidence of pulmonary metastasis was 8.0%-26.7%[8,9], accounting for roughly half of all recurrences/metastases in liver transplant recipients[10]. Nevertheless, the underlying mechanism of pulmonary metastasis after LT is elusive.
Recently, the impact of inflammation on cancer metastasis has received increasing attention. Previous studies showed that lung inflammation promoted pulmonary metastasis of several types of tumors[11-14]. Inflammation-induced lung metastasis could be attenuated by aspirin or antibiotics[12,15]. The global incidence of early postoperative infections is estimated to be over 20%. Among them, pneumonia is the predominant form for LT recipients[16,17]. Because of transplant-specific risk factors such as immunosuppression, the incidence of pneumonia is much higher in LT recipients than patients receiving partial hepatectomy (8%-48% vs. 2%-5%)[18,20]. Therefore, in this study, we aimed to assess the association between pneumonia and HCC pulmonary metastasis after LT and to elucidate the possible mechanisms.
METHODS
After excluding patients with incomplete information, loss to follow-up, or perioperative death, 234 adult HCC patients receiving primary LT from donation after citizens’ death from January 2015 to February 2019 in our center were enrolled as a training cohort, and another independent cohort of 179 subjects from Shulan Hospital Affiliated to Zhejiang Shuren University between 2017 to 2019 was designated for further validation of the predictive model. 212 patients in the training cohort and 163 patients in the validating cohort were within Hangzhou Criteria as we previously reported[5]. All cases were in accordance with the Regulations on Human Organ Transplant and national legal requirements. No organs from executed prisoners were used. Patients received triple immunosuppressant therapy incorporating tacrolimus, mycophenolate, and a steroid, as we previously reported[21]. This study complies with the guidelines of China Ethical Committee and the Declaration of Helsinki. Informed consents were obtained from all participants. Demographic and pathological information including cirrhosis, Child-Pugh score, and ischemia time were collected retrospectively. Based on their microscopic appearance, the pathologist classified HCC into four grades: well-differentiated (Grade 1), moderately differentiated (Grade 2), poorly differentiated (Grade 3), or undifferentiated (Grade 4). Pneumonia was diagnosed based on chest imaging showing infiltrates, accompanied by fever (temperature > 38.3 °C or < 36 °C), leukocytosis (white blood cell count > 10 or < 4 g/L), and clinical symptoms such as a new or worsening cough, dyspnea, and purulent secretions[22]. To exclude the possibility that pneumonia was induced by lung metastatic tumors, pneumonia in this study was defined as a lung infection that occurred before the detection of metastasis. Pulmonary metastasis was suspected by imaging and finally confirmed by pathology.
For the mouse model, six-week-old male Balb/c mice were used for the experiments. Each experimental group comprised ten randomly assigned animals. All animals were treated humanely in accordance with the guidelines detailed in the “Guide for the Care and Use of Laboratory Animals” by the National Academy of Sciences, as published by the National Institutes of Health. Institutional and national guidelines for the care and use of laboratory animals were followed. Mouse pneumonia model was induced as reported in[11]. 1 × 106 H22 cells in 100 μL PBS were injected into the tail vein, and immediately thereafter, 20 μg of LPS in 50 μL PBS were intra-nasally administered. Controls received PBS only. To deplete macrophages, clodronate liposomes (1.4 mg/20 g body weight) or an equal volume of PBS liposomes were injected intraperitoneally every other day. Vatalanib was administrated by gavage at a dose of 50 mg/kg per day as recommended[23]. Mice were sacrificed and lung tissues were fixed in 4% formaldehyde.
Hematoxylin-eosin staining
Preparation of lung sections and histopathological techniques was performed according to standard protocols[24]. Paraffin-embedded tissues were sectioned at 4 μm for histological and immunohistochemical analysis. The sections were stained with hematoxylin-eosin and evaluated under a microscope for histological examination. Two independent observers performed all histological assessments in a blinded fashion.
Immunocytochemistry
Cells were seeded on slides, incubated for 24 h, and then fixed with paraformaldehyde for 30 min at 4 °C. After rinsing three times in PBS, nonspecific proteins were blocked with serum for 30 min at room temperature. The cells were then incubated overnight at room temperature with the anti-VEGF receptor 2 antibody and subsequently washed twice in PBS. Following this, the cells were incubated for 1 hour with goat anti-rabbit immunoglobulin G-conjugated horseradish peroxidase diluted at 1:1,000, followed by a PBS wash. Finally, the cells were stained with diaminobenzidine.
Immunohistochemistry, and immunofluorescence staining
Specimens were sliced into 4 μm sections, de-waxed with xylene, and rehydrated using graded ethanol. Antigen retrieval was conducted in a microwave at 95 °C for 20 min, followed by cooling to room temperature. To block nonspecific binding sites, sections were treated with 5% bovine serum albumin (BSA) for 1 h. Subsequently, the sections were incubated with anti-VEGF and anti-CD31 antibodies. Representative fields were selected from each section.
Cell culture
The human HCC cell lines Huh-7 and SK-Hep-1, mononuclear cell line THP-1, lung fibroblasts HFL-1 and epithelium-derived lung adenocarcinoma cell line A549 were cultured in the supplier-recommended complete growth medium. All cells were maintained at 37 °C in a humidified incubator with 5% CO2. To induce monocyte-macrophage differentiation, THP-1 cells were treated with 150 nM phorbol 12-myristate 13-acetate (PMA) for 48 h. Cells were washed with cold PBS and then cultured with medium containing 50 ng/mL LPS or an equal volume of PBS for another 48 h. The supernatant was filtered and centrifuged at a speed of 3,000 g to remove cells and cell debris. The supernatants (LPS-conditioned and Control-conditioned) were then used to culture HCC cells for 48h. The VEGFR2 inhibitor Vatalanib[25,26], PI3K inhibitor LY294002[27,29], and AKT inhibitor MK-2206 2HCl[30] were provided by Sellleck (Selleck Chemicals, Houston, TX, USA), and used at recommended concentrations.
ELISA
The concentration of VEGF in the supernatant was measured using the Human ELISA Kit (Raybiotech, ELH-VEGF-1), following the manufacturer's instructions. Results were read at 450 nm with an Absorbance Reader (BIO-TEK ELX800). Each sample was tested in triplicate, and the average value was used for analysis.
Western blot
Total proteins were extracted from cells. Anti-β-actin antibody (Abcam, ab8226, 1:2,000),
Tumor migration and invasion assay
For migration assay, polycarbonate membrane transwell and Matrigel were used. A total of 1 × 104 HCC cells were suspended in 200 μL of serum-free medium and seeded into the upper chamber. The lower chamber was added with 700 μL of MEM containing 10% FBS. For invasion assay, 40 μL 1:8 diluted Matrigel was placed into the inserts and medium was removed without disturbing the layer of Matrigel on the membrane after rehydration. After incubation at 37 °C for the specified duration, cells remaining on the upper surface were removed using cotton swabs. The membranes were then fixed in paraformaldehyde and stained with 0.5% crystal violet. Cells on the lower surface were counted in randomly selected fields under a microscope (100X). The experiments were repeated independently three times.
Cell cycle and apoptosis assay
For cell cycle analysis, cells were harvested and fixed in ice-cold 75% ethyl alcohol at 4 °C overnight. After incubation with DNA PREP kit solution in the dark for 30 min, the cell cycle was detected by FACS and analyzed using ModFit LT 3.1 software. Apoptosis was assessed using an FITC Annexin V Apoptosis Detection Kit II and analyzed with a flow cytometer. All experiments were performed in triplicate.
Statistical analysis
Continuous variables were analyzed using Student's t-test, while categorical variables were evaluated using Pearson's chi-square test. Univariate analysis was performed for the potential prognostic factors, and the parameters that exhibited statistical significance were then included in multivariate logistic regression to identify the most critical characteristics of pulmonary metastasis. SPSS for Windows version 22.0 was used for all statistical analyses, and p value less than 0.05 was considered statistically significant. R software (version 4.0.2) was applied to integrate independent predictors of pulmonary metastasis into a nomogram in the training cohort. Propensity scores were estimated using a logistic model with the SAS software package 9.4.
RESULTS
Pneumonia independently increased the pulmonary metastasis risk in liver transplant recipients
For the training cohort, the median follow-up time was 31.6 months (ranging from 8 to 55 months). Among 75 patients with recurrence/metastasis, 48 had pulmonary metastasis (16 had sole pulmonary metastasis, 32 had both pulmonary and other site recurrence/metastasis, including liver graft, bone and other sites). As seen in Table 1 and [Supplementary Table 1], Patients with pneumonia had a higher rate of pulmonary metastasis when compared with recipients without pneumonia (38.6% vs. 10.6%,
Clinicopathological features of LT recipients
| Pneumonia (-) | Pneumonia (+) | P value | ||
| Gender | male | 131 | 78 | 0.087 |
| female | 20 | 5 | ||
| Age | 52.61 ± 9.23 | 52.89 ± 9.04 | 0.826 | |
| HBV | - | 13 | 3 | 0.148 |
| + | 138 | 80 | ||
| Cirrhosis | - | 6 | 2 | 0.715 |
| + | 145 | 81 | ||
| Tumor number | single | 66 | 30 | 0.270 |
| multiple | 85 | 53 | ||
| Diameter (cm) | 5.22 ± 4.52 | 6.41 ± 5.2 | 0.330 | |
| WIT (min) | 9.63 ± 7.49 | 8.77 ± 5.89 | 0.335 | |
| CIT (h) | 9.43 ± 3.28 | 9.52 ± 3.06 | 0.824 | |
| AFP level (ng/mL) | 4028.6 ± 14074.1 | 3344.8 ± 11683.1 | 0.708 | |
| ICU stay (days) | 222.7 ± 104.7 | 242.6 ± 179.7 | 0.359 | |
| MELD score | 15.61 ± 10.49 | 14.96 ± 9.27 | 0.640 | |
| MVI | - | 91 | 53 | 0.689 |
| + | 60 | 30 | ||
| HCC Differentiation | 1 | 17 | 11 | 0.971 |
| 2 | 78 | 41 | ||
| 3 | 54 | 30 | ||
| 4 | 2 | 1 | ||
| TMN stage | I | 46 | 25 | 0.932 |
| II | 49 | 24 | ||
| III | 53 | 32 | ||
| IV | 3 | 2 | ||
| Tacrolimus (ng/mL) | 7.94 ± 2.51 | 7.71 ± 2.46 | 0.791 | |
| Pulmonary metastasis | - | 135 | 51 | < 0.001 |
| + | 16 | 32 |
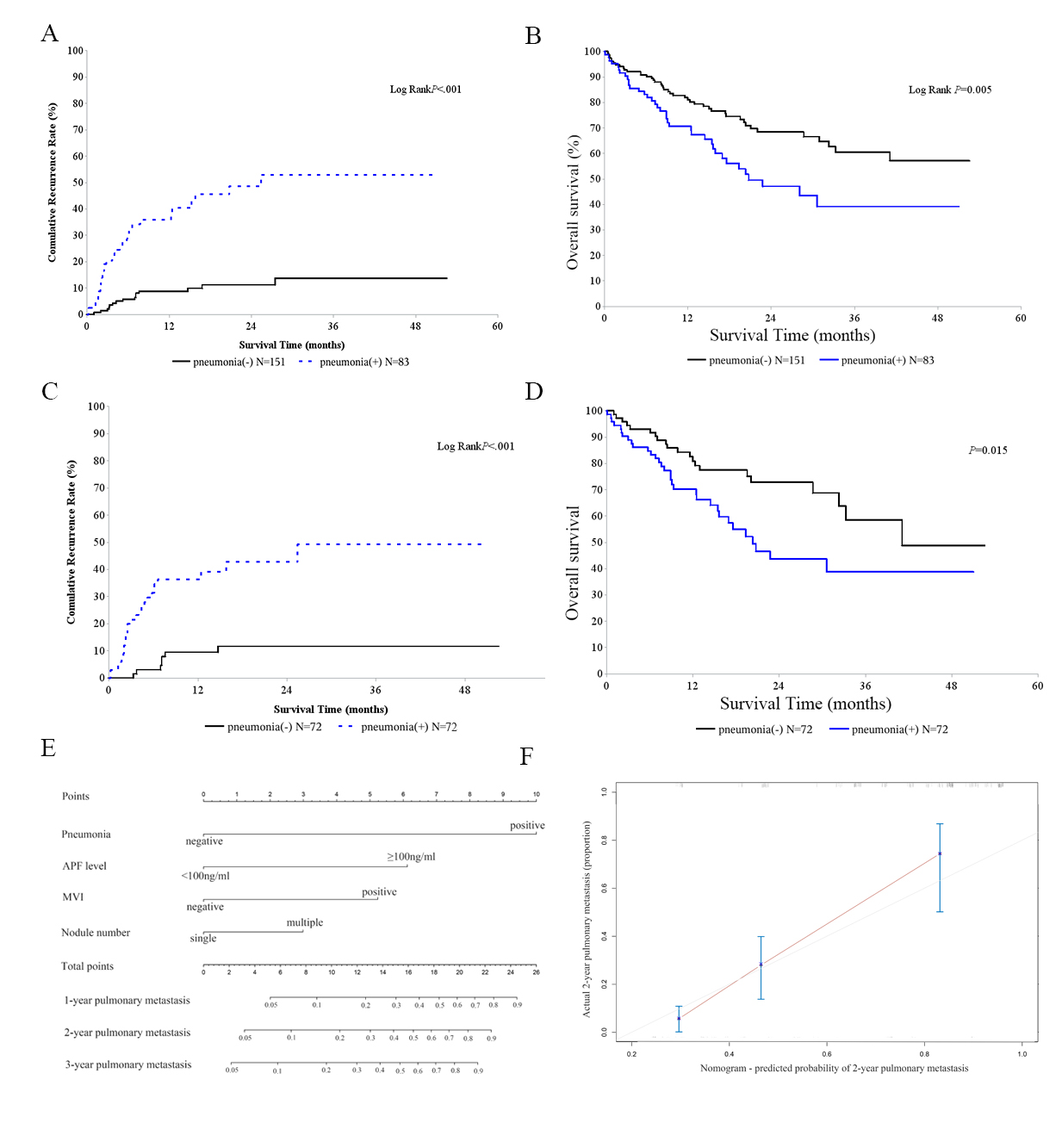
As shown in Table 2, tumor number, AFP level, microvascular invasion (MVI), and pneumonia were significantly associated with pulmonary metastasis and overall survival (P < 0.05). History of ICU stay was correlated only with recipients’ overall survival (P < 0.05). Multivariate analysis showed that history of ICU stays, AFP level, and pneumonia impacted overall survival. AFP level, MVI, and pneumonia were independent risk factors for pulmonary metastasis [Table 3]. Recipients with post-LT pneumonia exhibited an increase in pulmonary metastasis (P <0.001, Figure 1A) and a significant reduction in overall survival
Figure 1. Pneumonia increases pulmonary metastasis and nomogram incorporating risk factors is effective. Liver transplantation recipients for HCC with pneumonia had increased pulmonary metastasis (A) and decreased overall survival (B). After PSM, two paired groups of patients were formed, with 72 subjects in each. Recipients with pneumonia showed increased pulmonary metastasis (C) and shortened overall survival (D); Nomogram incorporating risk factors predicted pulmonary metastasis for LT recipients (E) and was externally validated in an independent cohort (C-index 0.794, F)
Univariate analysis for overall survival and pulmonary metastasis
| Overall survival | Pulmonary metastasis | |||
| Hazard Ratio | P value | Hazard Ratio | P value | |
| Age | 1.018 | 0.170 | 0.991 | 0.587 |
| MELD score | 1.017 | 0.078 | 1.013 | 0.329 |
| ICU stay | 1.002 | 0.009 | 0.998 | 0.295 |
| Tumor number | 1.897 | 0.012 | 2.308 | 0.012 |
| Tumor diameter | 0.998 | 0.543 | 1.001 | 0.127 |
| Tumor Differentiation | 0.900 | 0.114 | 0.853 | 0.087 |
| AFP level | 2.204 | < 0.001 | 2.644 | < 0.001 |
| CHILD score | 1.089 | 0.074 | 1.023 | 0.724 |
| WIT | 1.012 | 0.452 | 1.020 | 0.333 |
| CIT | 1.064 | 0.066 | 1.032 | 0.481 |
| SEX | 0.686 | 0.375 | 0.729 | 0.544 |
| Cirrhosis | 1.158 | 0.804 | 1.654 | 0.503 |
| HBV infection | 1.862 | 0.291 | NA | NA |
| MVI | 2.539 | < 0.001 | 2.215 | 0.007 |
| Pneumonia | 1.857 | 0.006 | 5.435 | < 0.001 |
Multivariable hazard ratios for overall survival and pulmonary metastasis
| Overall survival | Pulmonary metastasis | |||
| Hazard Ratio | P value | Hazard Ratio | P value | |
| ICU | 1.002 | 0.014 | NA | NA |
| AFP | 2.204 | < 0.001 | 2.308 | 0.007 |
| Tumor number | 1.274 | 0.376 | 1.035 | 0.079 |
| MVI | 1.886 | 0.008 | 2.806 | < 0.0001 |
| Pneumonia | 1.606 | 0.042 | 6.360 | < 0.0001 |
Nomogram constructed was effective in predicting pulmonary metastasis
Based on risk factors including pneumonia, a nomogram predicting pulmonary metastasis of LT recipients was constructed [Figure 1E], and this nomogram worked effectively (C-index 0.776,
Pneumonia promoted pulmonary metastasis of HCC cells in mice model
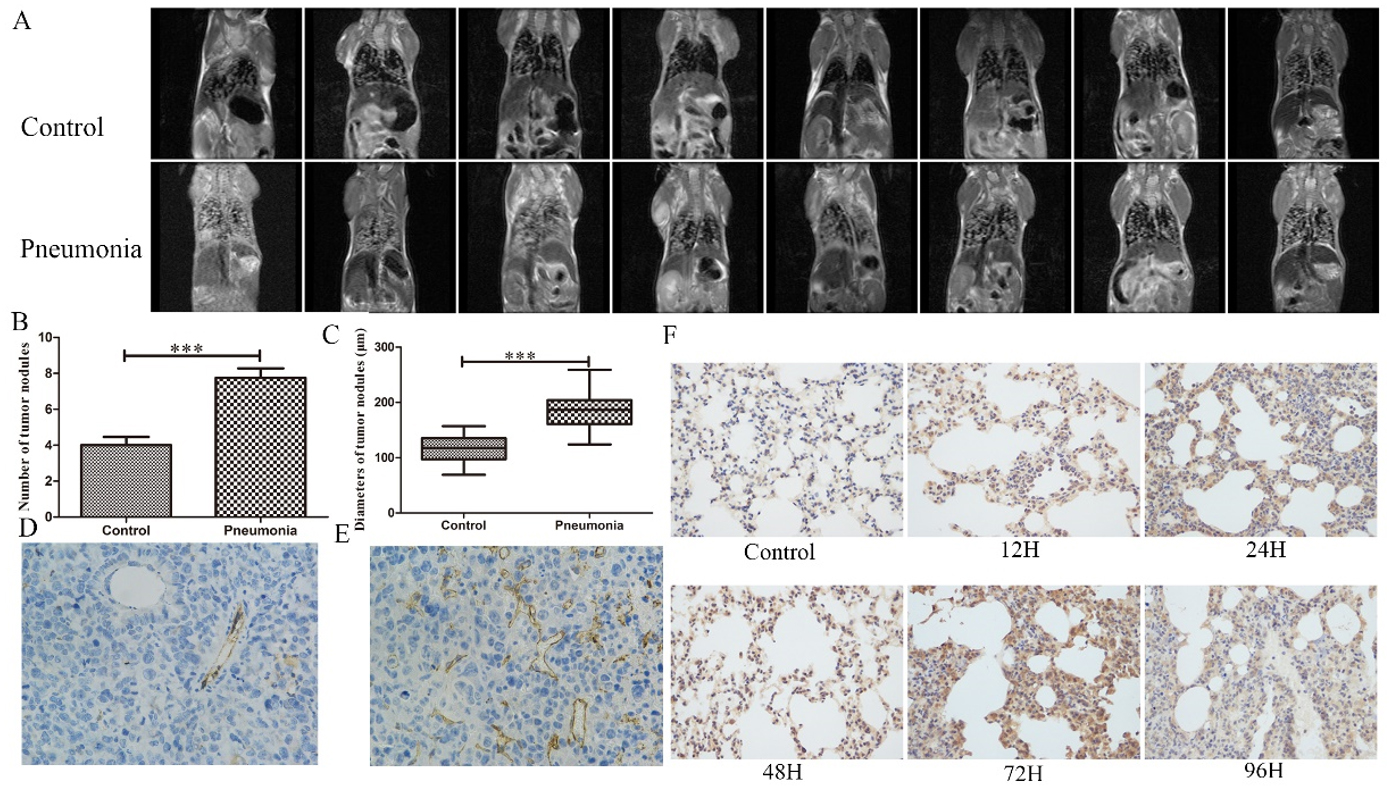
To better illustrate the association between pneumonia and pulmonary metastasis, we established an LPS-induced mouse pneumonia model [Supplementary Figure 2] and injected H22 cells into the tail vein to mimic the HCC recurrence. The preliminary experiment showed a median survival of 13 days of the H22 cells-injected mice. Therefore, we performed Magnetic Resonance Imaging 10 days after cell injection to evaluate the tumor growth in the lung. We found significantly exacerbated pulmonary metastasis in the pneumonia group as compared with control [Figure 2A]. The following pathology confirmed the results that pneumonia mice had higher tumor burden in the lung [Figure 2B and C].
Figure 2. Pneumonia facilitated HCC pulmonary metastasis and angiogenesis in mouse models. (A) MRI images of the control and pneumonia mice; Mean metastatic nodule number (B) and diameter (C); Representative images of CD31 staining of the metastatic nodules of control (D) and pneumonia group (E); (F) VEGF was upregulated consecutively in the pneumonia mice lungs for at least 96 hours following LPS instillation. ***: P < 0.001
Pneumonia increased VEGF secretion in pulmonary macrophages
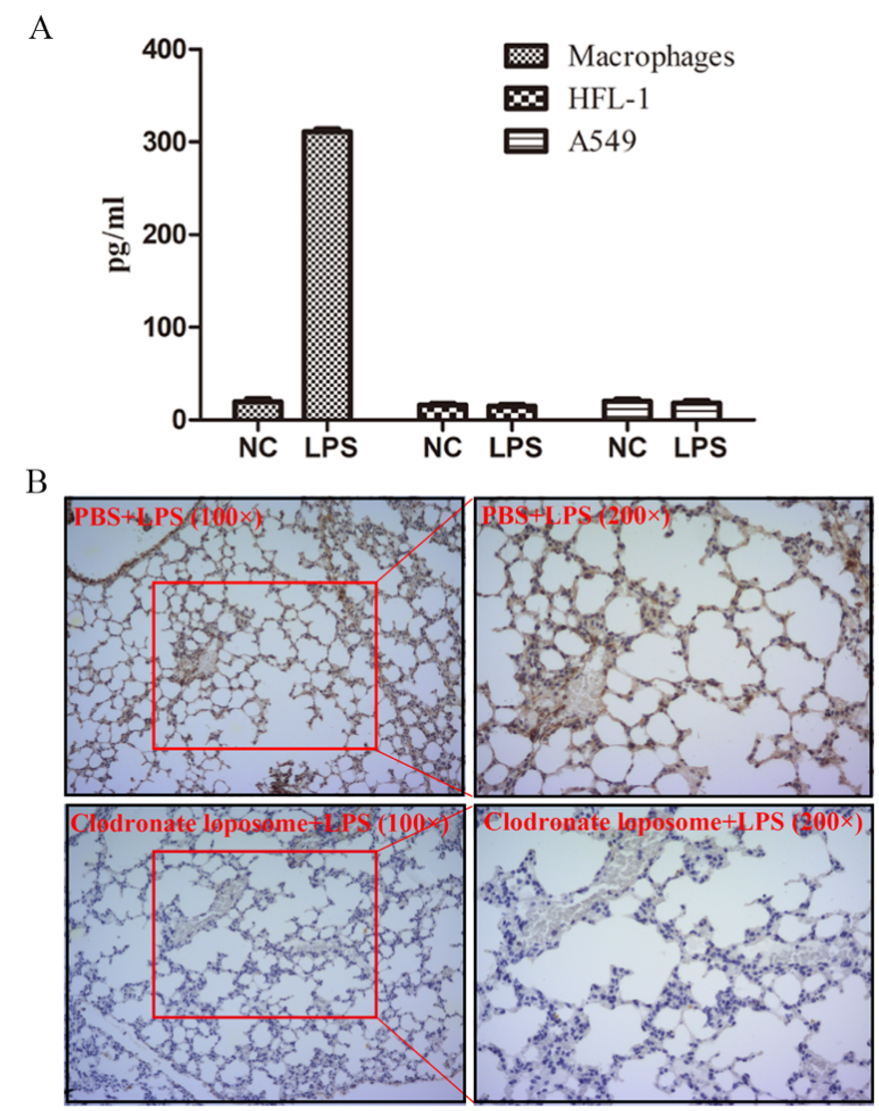
Since angiogenesis is a key process in regulating tumor metastasis[31-33], we assessed the microvessel density using CD31 staining and found significantly higher CD31 intensity in the pneumonia group compared with control [Figure 2D and E]. We further examined the expression of VEGF and TGF-β, cytokines regulating angiogenesis. In the mice lung, we found a time-dependent elevated VEGF level after LPS stimulation [Figure 2F], while the intensity of TGF-β remained almost constant [Supplementary Figure 3]. To reveal the origin of VEGF, we tested the concentration of VEGF in the supernatants of THP-1-derived macrophages, lung fibroblasts HFL-1, and lung epithelium-derived cell line A549 stimulated with LPS or not. Although they produced comparable levels of VEGF in normal conditions, when stimulated with LPS, only THP-1-derived macrophages produced dramatically elevated VEGF [Figure 3A]. Consistently, in vivo, macrophage-depleted mice [Supplementary Figure 4] showed significantly decreased VEGF intensity in the lungs when instilled with LPS [Figure 3B]. Taken together, VEGF was mainly originated from macrophages following LPS instillation.
Figure 3. VEGF was originated from macrophage. (A) Concentration of VEGF in supernatants of THP-1-derived macrophages, lung fibroblast cell line HFL-1, and lung epithelium-derived adenocarcinoma cell line A549 stimulated with LPS or not; (B) Immunohistochemistry staining of VEGF of lungs in the control or macrophage-depleted mice.
Macrophage-derived VEGF promotes carcinogenesis via inhibition of apoptosis through PI3K/AKT/Cas-9 signaling besides angiogenesis
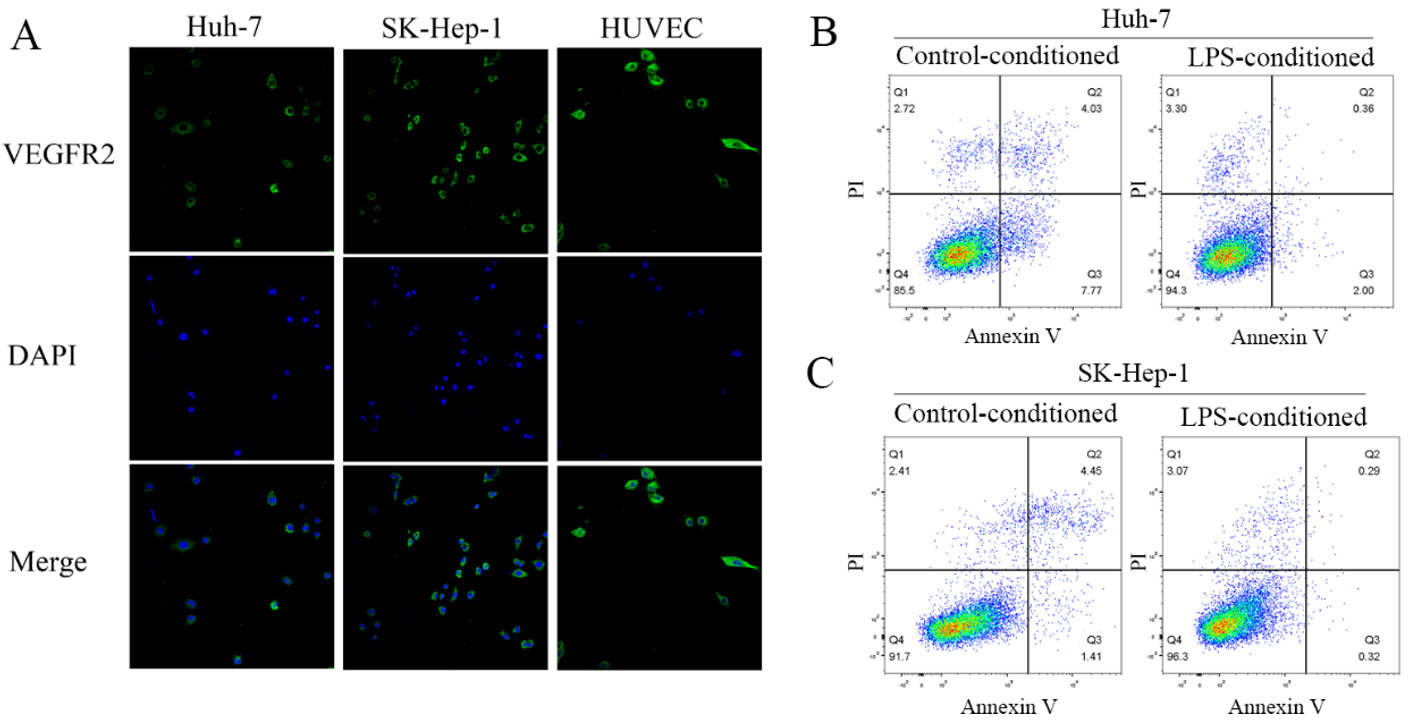
We found HCC cells expressed abundant VEGF receptor 2 (VEGFR2) [Figure 4A]. When cocultured with LPS-conditioned medium, HCC cells presented significantly reduced cell apoptosis [Figure 4B], although there were no significant changes in proliferation, migration, or invasion after co-culturing
Figure 4. LPS-conditioned medium ameliorated HCC cell apoptosis. (A) Immunofluorescence staining of VEGFR2 for HCC cell lines Huh-7 and SK-Hep-1, with human umbilical vein endothelial cells as positive control; LPS-conditioned medium inhibited apoptosis of the Huh-7 (B) and SK-Hep-1 (C) cells.
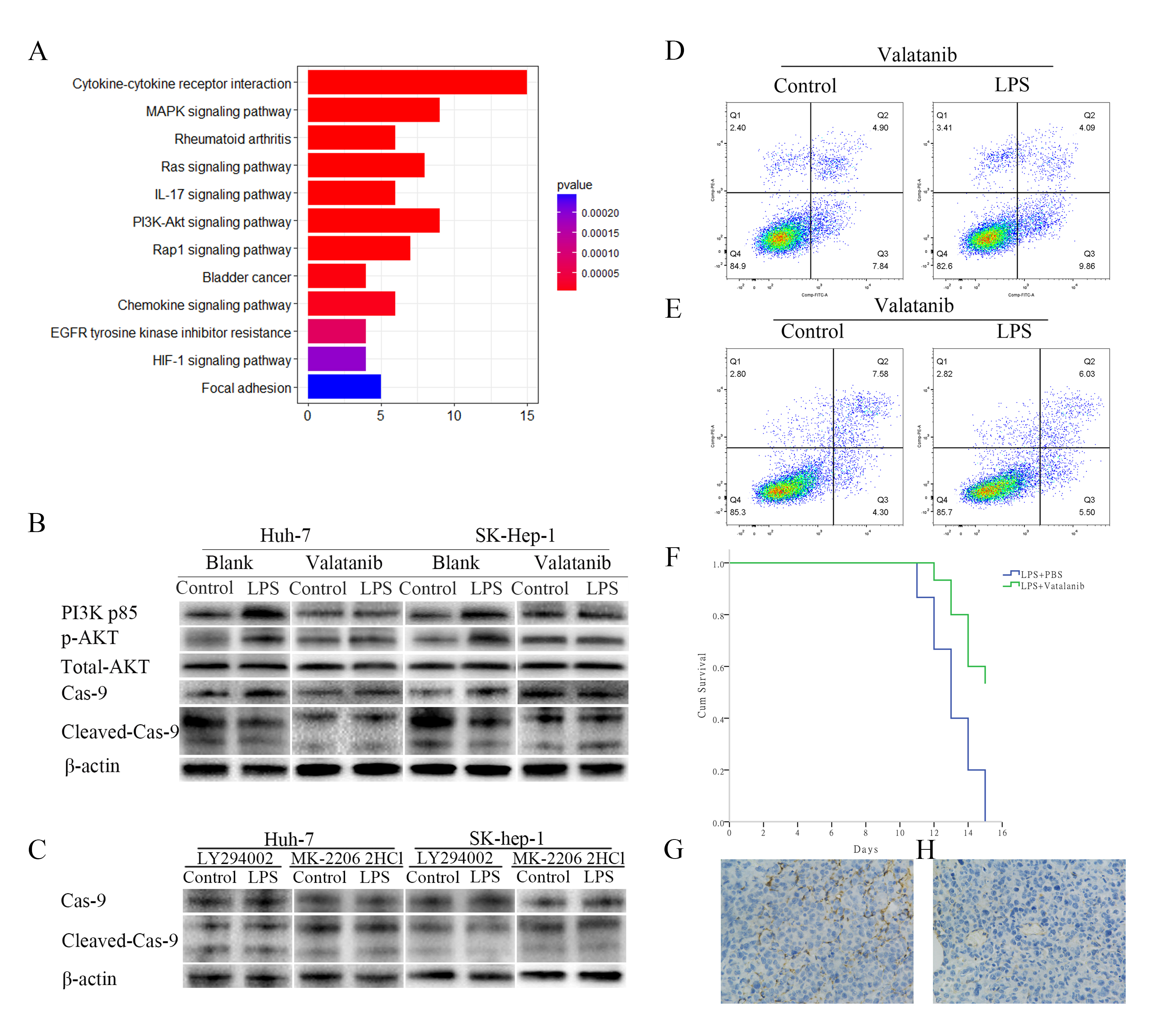
Figure 5. LPS-conditioned medium activated PI3K/AKT/Cas-9 signaling and prophylactic administration of Vatalanib improved the prognosis of the pneumonia mice. (A) KEGG analysis based on cytokine array; (B) Western blot of PI3K/AKT signaling of HCC cells cultured in the Control-conditioned and LPS-conditioned medium; (C) Activation of the PI3K/AKT signaling was abrogated with PI3K inhibitor LY294002 or AKT inhibitor MK-2206 2HCl; Changes in apoptosis of Huh-7 (D) and SK-Hep-1 (E) was abrogated by Vatalanib; (F) Cumulative survival of the pneumonia mice administered with PBS or Vatalanib (P < 0.05); CD31 staining of the metastatic nodules of the pneumonia mice administered with PBS (G) or Vatalanib (H).
In vivo, Vatalanib administration dramatically improved the prognosis of the pneumonia mice [Figure 5E]. No obvious adverse effects such as diarrhea were observed in mouse model. Moreover, microvessel density in the metastatic nodules was also decreased [Figure 5F and G].
DISCUSSION
The ambiguity and complexity of pulmonary metastasis make it difficult to predict or intervene. To the best of our knowledge, this is the first study to evaluate the impact of post-LT pneumonia on HCC metastasis. We found that pneumonia was a very common phenomenon after LT and an independent risk factor for pulmonary metastasis based on two independent cohorts from different LT centers. On the one hand, pneumonia itself is one of the leading causes of sepsis and mortality after liver transplantation[18]. On the other hand, it increases the risk of pulmonary metastasis. Therefore, we should take intensive care of the post-LT recipients to prevent pneumonia. For the recipients already with pneumonia, close follow-up will be needed.
Hospital-acquired pneumonia early following LT is predominantly caused by Gram-negative bacteria. This group of pathogens induce pneumonia through the secretion of LPS. Therefore, the LPS-induced mouse pneumonia model could fully imitate the hospital-acquired pneumonia of the LT recipients during their hospital stay. In this study, not only clinical investigation but also in vivo models proved that pneumonia promotes HCC pulmonary metastasis. Local inflammation facilitates tumor metastasis through various mechanisms, such as triggering the formation of neutrophil extracellular traps and enhancing adhesion capacity at the metastatic site[14,34,35]. As is well known, angiogenesis plays a pivotal role in tumor metastasis[36-38]. We found that microvessel density in the metastatic nodules of pneumonia mice was significantly elevated. Furthermore, VEGF, the key angiogenesis modulator, was remarkably upregulated following LPS instillation.
For VEGF, there are abundant existing studies concerning the pro-angiogenesis effect of VEGF[39,40]. It is capable of leading to leaky vascular networks that facilitate tumor cell invasion and induce EMT[41,42]. In this study, we found that macrophage-derived VEGF may inhibit apoptosis by activating PI3K/AKT/Cas-9 signaling. Elevation of VEGF induced by pneumonia in the lung may thus foster a pro-metastatic niche. The high incidence of pneumonia in patients receiving LT may partially explain why lung metastasis is more prevalent compared with liver resection or locoregional therapies. Anti-angiogenesis agents targeting the VEGF/VEGF receptor pathway have become a crucial component of standard therapy for various cancer types[43,45], so we explored the potential of preventing pulmonary metastasis through the administration of VEGFR2-specific inhibitor Vatalanib[25,46,47]. We found that prophylactic administration of VEGFR2 inhibitor Vatalanib could ameliorate HCC pulmonary metastasis for the pneumonia mice. As this therapeutic effect was only observed in a mouse model with a small sample size, it is only a hypothesis and further validation is needed.
Immunosuppressants could also influence HCC recurrence after live transplantation. Limiting the use of CNI and using m-TOR inhibitors could help reduce HCC recurrence rates[48]. However, owing to differences in metabolic rates, equal administration of immunosuppressants could result in different plasma concentrations in mice. Therefore, immunosuppressants were not used in the mouse model.
The primary limitation of this study is that the cohort enrolled is small, especially the number of recipients with pulmonary metastasis. We hope to validate this as the number of transplantations increases. It is also limited as the mouse pneumonia model, which was not immunosuppressed, could not adequately reflect the clinical practice.
In summary, this study provided a novel explanation for the extremely high incidence of pulmonary metastasis after LT. We strongly recommend an early screening and prompt treatment of pneumonia in the perioperative period in HCC patients. Anti-angiogenesis agents that target the VEGF/VEGF receptor pathway might be a promising strategy in liver recipients with pneumonia for reducing HCC pulmonary metastasis risk.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the study: Zhuang R, Wei Q, Xu X
Performed data analysis and interpretation: Dong S, Lu D
Performed data acquisition, in vivo and in vitro experiments: Zhuang R, Zhuo J
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by Grant Zhejiang Provincial Natural Science Foundation of China (LQ22H160052); National Natural Science Foundation of China (82300742); The Major Research Plan of the National Natural Science Foundation of China (92159202).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Research involving human subjects, human material, or human data was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of The First Affiliated Hospital of Zhejiang University School of Medicine. Ethical approval and consent to participate Approval was provided by the Clinical Research Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (2020-852). Informed consent was obtained from each subject prior to their participation in this study.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-128.
2. Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072-9.
3. Han S, Kim G, Ko JS, et al. Safety of the use of blood salvage and autotransfusion during liver transplantation for hepatocellular carcinoma. Ann Surg. 2016;264:339-43.
4. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-9.
5. Xu X, Lu D, Ling Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035-41.
6. Désert R, Rohart F, Canal F, et al. Human hepatocellular carcinomas with a periportal phenotype have the lowest potential for early recurrence after curative resection. Hepatology. 2017;66:1502-18.
7. Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new moral to the story. Ann Surg. 2017;265:557-64.
8. Gao Q, Zhou J, Wang XY, et al. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Ann Surg Oncol. 2012;19:455-66.
9. Agopian VG, Harlander-Locke M, Zarrinpar A, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-27.
10. Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266:118-25.
11. El Rayes T, Catena R, Lee S, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci U S A. 2015;112:16000-5.
12. Nishimura E, Fukuda K, Matsuda S, et al. Inhibitory effect of aspirin on inflammation-induced lung metastasis of cancer cells associated with neutrophil infiltration. Surg Today. 2023;53:973-83.
13. Pooladanda V, Thatikonda S, Priya Muvvala S, Godugu C. Acute respiratory distress syndrome enhances tumor metastasis into lungs: Role of BRD4 in the tumor microenvironment. Int Immunopharmacol. 2023;115:109701.
14. Gowing SD, Chow SC, Cools-Lartigue JJ, et al. Gram-negative pneumonia augments non-small cell lung cancer metastasis through host toll-like receptor 4 activation. J Thorac Oncol. 2019;14:2097.
15. Le Noci V, Guglielmetti S, Arioli S, et al. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep. 2018;24:3528-38.
16. Angarita SAK, Russell TA, Kaldas FM. Pneumonia after liver transplantation. Curr Opin Organ Transplant. 2017;22:328-35.
17. Pirat A, Ozgur S, Torgay A, Candan S, Zeyneloğlu P, Arslan G. Risk factors for postoperative respiratory complications in adult liver transplant recipients. Transplant Proc. 2004;36:218-20.
18. Weiss E, Dahmani S, Bert F, et al. Early-onset pneumonia after liver transplantation: microbiological findings and therapeutic consequences. Liver Transpl. 2010;16:1178-85.
19. Mathur AK, Ghaferi AA, Osborne NH, et al. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010;14:1285-91.
20. Rayya F, Harms J, Bartels M, Uhlmann D, Hauss J, Fangmann J. Results of resection and transplantation for hepatocellular carcinoma in cirrhosis and noncirrhosis. Transplant Proc. 2008;40:933-5.
21. Ling Q, Xie H, Lu D, et al. Association between donor and recipient TCF7L2 gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation in a Han Chinese population. J Hepatol. 2013;58:271-7.
22. Lui JK, Spaho L, Holzwanger E, et al. Intensive care of pulmonary complications following liver transplantation. J Intensive Care Med. 2018;33:595-608.
23. Wood JM, Bold G, Buchdunger E, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178-2189.
24. Ling Q, Xu X, Ye P, et al. The prognostic relevance of primary tumor location in patients undergoing resection for pancreatic ductal adenocarcinoma. Oncotarget. 2017;8:15159-67.
25. Zhong H, Wang D, Wang N, et al. Combinatory action of VEGFR2 and MAP kinase pathways maintains endothelial-cell integrity. Cell Res. 2011;21:1080-7.
26. Li J, Cubbon RM, Wilson LA, et al. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Cell Res. 2011;108:1190-8.
27. Mbengue A, Bhattacharjee S, Pandharkar T, et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683-7.
28. Bolen CR, Ding S, Robek MD, Kleinstein SH. Dynamic expression profiling of type I and type III interferon-stimulated hepatocytes reveals a stable hierarchy of gene expression. Hepatology. 2014;59:1262-72.
29. Gardner EE, Connis N, Poirier JT, et al. Rapamycin rescues ABT-737 efficacy in small cell lung cancer. Cancer Res. 2014;74:2846-56.
30. Yang J, Wu Z, Renier N, et al. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell. 2015;160:161-76.
31. Bonapace L, Coissieux MM, Wyckoff J, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130-3.
32. Song R, Song H, Liang Y, et al. Reciprocal activation between ATPase inhibitory factor 1 and NF-κB drives hepatocellular carcinoma angiogenesis and metastasis. Hepatology. 2014;60:1659-73.
33. Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512-26.
34. Qi JL, He JR, Liu CB, et al. Pulmonary Staphylococcus aureus infection regulates breast cancer cell metastasis via neutrophil extracellular traps (NETs) formation. 2020;1:188-201.[PMID:34766117 DOI:10.1002/mco2.22 PMCID:PMC8491238] Caution! MedComm (2020). 2020;1:188-201.
35. Xia X, Zhang Z, Zhu C, et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13:1017.
36. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409-25.
37. Lin ZY, Chen G, Zhang YQ, et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol Cancer. 2017;16:48.
38. Ling CC, Ng KT, Shao Y, et al. Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J Hepatol. 2014;60:103-9.
39. von Marschall Z, Cramer T, Höcker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48:87-96.
40. Feng Q, Zhang C, Lum D, et al. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun. 2017;8:14450.
41. Yang X, Zhang Y, Hosaka K, et al. VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci U S A. 2015;112:E2900.
42. Kong D, Zhou H, Neelakantan D, et al. VEGF-C mediates tumor growth and metastasis through promoting EMT-epithelial breast cancer cell crosstalk. Oncogene. 2021;40:964-79.
43. Inoue K, Torimura T, Nakamura T, et al. Vandetanib, an inhibitor of VEGF receptor-2 and EGF receptor, suppresses tumor development and improves prognosis of liver cancer in mice. Clin Cancer Res. 2012;18:3924-33.
44. Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465-77.
45. Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518-29.
46. Lee B, Clarke D, Al Ahmad A, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005-23.
47. Lee H, Lee JK, Park MH, et al. Pathological roles of the VEGF/SphK pathway in Niemann-Pick type C neurons. Nat Commun. 2014;5:5514.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].