Carbon-based materials: adsorptive removal of antibiotics from water
Abstract
The contamination generated by multiple antibiotics represents a general concern given its impact at the environmental level, mainly affecting the planet’s soil and water and impacting the development of numerous species. Additionally, a new problem has been triggered in terms of the development of antibiotic-resistance genes in various pathogenic microorganisms generating concern for the health sector in terms of the efficiency of antibiotics in the future. These actual problems and concerns demand efforts and actions to remove or eliminate these contaminants. Multiple alternatives to reduce the impact of antibiotics in water have been carried out, such as advanced oxidation, reverse osmosis, and membrane filtration. However, adsorption techniques have presented more favorable and viable results in which carbon-based materials are an efficient tool to remediate the environment that can take advantage of other alternatives due to their characteristics. This review presents different carbon-based absorptive materials such as biochar, carbon nanotubes, activated carbon, and graphene to remove these contaminants, given their characteristics and favorable results. However, process integration, production, and modification continue to be challenging and require more research and experimentation.
Keywords
INTRODUCTION
The release of antibiotics into the environment has been a concerning issue since the discovery of antibiotic-resistant bacteria and antibiotic-resistance genes in the last half of the 19th century. These compounds normally end up in soil and water bodies, presenting a risk to ecosystem equilibrium[1-3]. Moreover, antibiotics can be toxic and detrimental to human and animal health, provoking a lack of response to an infection treated with regular antibiotics treatment. The dissemination of this type of drug might affect natural microbial communities, presenting a bactericidal or bacteriostatic effect. Considering microorganisms are vital to most biogeochemical cycles, they can alter nitrogen transformation, methanogenesis, sulfate reduction, nutrient cycling, and organic matter degradation[4,5].
There are several means by which antibiotics and pharmaceutically related active residues reach water bodies, the most common being the excretion by animals and humans of unaltered molecules that were not metabolized in the form of urine and feces[2,6-8]. In agriculture and aquaculture, antibiotics protect from bacterial infections, ensuring crop growth. Other contamination sources include the leaching from discarded medications and the discharge of waste from hospitals and industries[9]. Sulfonamide antibiotics (sulfamethoxazole, sulfamethazine, and sulfamethoxy-pyridazine) were found in the groundwater of northeastern China’s Limin along with macrolides (Lincomycin hydrochloride; Cefaclor), chloramphenicol (Florfenicol) and β-lactams (Ampicillin sodium). These with varying concentrations depending on surface proximity, river, and sources of pollution nearby[10]. In another study performed in Romanian groundwater resources, several antibiotic compounds were found and quantitated at values ranging from below the detection threshold to 917 ng/L for cefepime. Trimethoprim, macrolide, sulfonamide, and β-lactams were the most prevalent antibiotic classes, followed by tetracyclines and fluoroquinolones[6,8,11]. In Table 1, information on common antibiotic contaminants is summarized[12-14]. Under this premise, various approaches have emerged to remove this contamination. Among those, carbon materials are becoming a promising substitute for the current lacking technology as they present good stability, tunability, and physio-chemical properties[15-24]. Moreover, the adsorption method is one of the most appealing approaches due to its simple operation, high efficiency, cost-effectiveness, and low environmental impact[25].
Physicochemical, structural, and pharmacokinetic data of different antibiotics. Data was extracted from Refs.[12-14]
| Antibiotics | Drug class | Structure | Formula | Molar mass | CAS number | Elimination half-life | Excretion | Chemical safety |
| Ampicillin | Aminopenicillins | 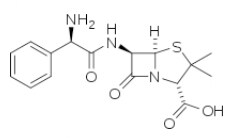 | C16H19N3O4S | 349.41 g·mol-1 | 69-53-4 | Approx. 1 h | 75 to 85% renal |  |
| Ciprofloxacin | Quinolones | 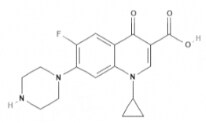 | C17H18FN3O3 | 331.34 g·mol-1 | 85721-33-1 | 3 to 4.8 h | 27 to 46% urinary |  |
| Tylosin | Macrolides | 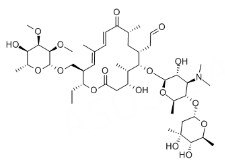 | C46H77NO17 | 916.1 g·mol-1 | 1401-69-0 | Approx. 20.46 h | 11% (in sheep) |  |
| Levofloxacin | Quinolones | 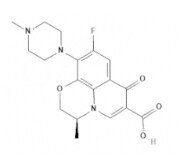 | C18H20FN3O4 | 361.4 g·mol-1 | 100986-85-4 | 6 to 8 h | 87% urinary |  |
| Trimethoprim | Urinary anti-infectives | 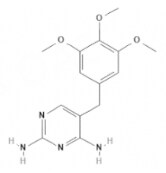 | C14H18N4O3 | 290.32 g·mol-1 | 738-70-5 | 2 to 3 h | 50% to 60% urinary |  |
| Sulfamethoxazole | Sulfonamides | 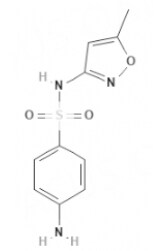 | C10H11N3O3S | 253 g·mol-1 | 723-46-6 | 7 to 12 h | 84.5% urinary | 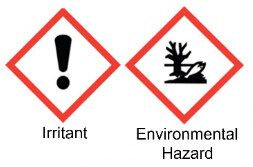 |
| Lincomycin | Lincomycin derivatives | 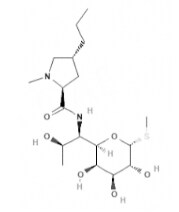 | C18H34N2O6S | 406.5 g·mol-1 | 154-21-2 | Approx. 5.4 h | 1.8 to 30.3% urinary | - |
| Cefaclor | Cephalosporins | 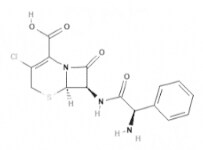 | C15H14ClN3O4S | 367.8 g·mol-1 | 53994-73-3 | 0.6 to 0.9 h | 60 to 85% urinary |  |
| Florfenicol | Amphenicols | 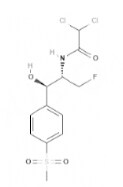 | C12H14Cl2FNO4S | 358.2 g·mol-1 | 73231-34-2 | - | - |  |
| Tetracycline | Tetracyclines | 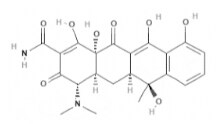 | C22H24N2O8 | 444.4 g·mol-1 | 60-54-8 | 6 to 12 h | - |  |
Carbon-based materials have demonstrated their efficacy in removing antibiotic molecules from water sources through their adsorptive properties. In this review, we have mainly focused on the most common types of carbon materials for combating contamination: graphene, carbon nanotubes (CNTs), activated carbon (AC), and biochar (BC). In each section, we present the main published studies of adsorption techniques to eliminate a particular antibiotic and their respective results.
Functional entities of carbon-based materials
Functional attributes of graphene
Graphene is a single-layer, two-dimensional sheet kept together by an interlocking network of sp2 hybrid bonds. It has a strong in-plane σC-C bond and an out-of-plane π bond. The 2p orbitals (that create the π state bands that delocalize over the sheet of carbons) confer the material’s fascinating properties as zero effective mass, a very high thermal conductivity (~5000 Wm-1 K-1), high mobility of charge carriers
It has a large surface-to-volume ratio and a strength as high as 130 GPa (assuming a thickness of about 0.335 nm) and is the strongest material currently in use. Graphene becomes nonlinearly elastic due to the fact that the amplitude of thermal rippling decreases nonlinearly with increasing tensile strain[28]. Also, graphene has a large specific surface area (2630 m2 g-1), high Young’s modulus (~1.0 TPa), excellent optical transmittance (~97.7%), good electrical conductivity, and the ability to withstand current densities of 108 A/cm2[26]. Other notable characteristics and suitability of graphene for remediation applications are shown in Figure 1.
Functional attributes of carbon nanotubes
The structure of carbon nanotubes, an sp2 hybridized allotrope of carbon, may be described as a rolled-up sheet of graphene, a planar hexagonal lattice of carbon atoms. Although functional attributes of carbon nanotubes (CNTs) display the greatest variety and richness of topologies and structure-property relationships, it has the simplest chemical composition and atomic bonding arrangement[29]. CNTs are available in three distinct geometries: armchair, zigzag, and chiral. The chirality governs the mechanical, electrical, optical, and other characteristics of CNTs[30].
CNTs may be single-walled carbon nanotubes (SWCNTs) or multi-walled carbon nanotubes (MCWNTs) [Figure 2], depending on how many layers there are in the CNTs. The structure of SWCNTs may be thought of as a layer of graphene wrapped around itself to form a tube, whereas MWNTs are made up of many rolled layers (concentric tubes) of graphene. Most SWCNTs range in diameter from 0.4 to > 3 nm, but they can also be several million times longer than wide. The interlayer diameter in MWCNTs is 3.4 Å; as for MWCNTs, it spans from 1.4 to at least 100 nm. The optical, thermophysical, and photo-thermal conversion capabilities of CNTs are all influenced by their morphologies[30].
Due to variations in length, helicity, thickness, number of layers, etc., CNTs manufactured of identical graphite sheets can have distinct mechanical, thermal, and electrical characteristics. Depending on the structural arrangements, they may be metallic or semiconducting. When a voltage is applied to the end of nanotubes in an armchair form, they act like metal, and current flows. The chiral and zigzag forms, which are the other two, function as semiconductors. CNTs have a tensile strength that is 50 times more than steel and a Young’s modulus that can be up to five times higher than steel. Due to their unique qualities and low density, CNTs are a possible choice for structural applications. While acting as an insulator lateral to the tube axis, CNTs are effective heat conductors along the tube. Thermal conductivity and low-temperature specific heat directly point to 1D quantization of the phonon band structure[29,31]. Other notable characteristics and suitability of CNTs for remediation applications are shown in Figure 3.
Functional attributes of biochar
Biochar has been utilized as an adsorbent for water contaminants because of its large specific surface area, porous structure, surface functional groups, and high mineral content [Figure 4]. Biochar is a solid compound produced by pyrolysis when heat energy evaporates biomass’s moisture and volatile components[32]. All biogenic materials may primarily be turned into biochar, charcoal being the most well-known variety of biochar. The raw material used to produce biochar affects the adsorption properties of the char. Previous research has shown that the raw biomass material, production method, and process parameters affect biochar properties[32]. The construction of the biochar pore structure and the emergence of primitive pores in biochar are influenced by water losses in the dehydration process and the release of volatile components from the carbon matrix during biomass pyrolysis. The pore structure of biochars consists to a large extent of micropores, which may account for more than 80% of the total pore volume or surface area. A large surface area is necessary for many biochar applications since it is associated with several other biochar features. The pores cover several orders of magnitude in biochar, which may be divided into micropores (2 nm), mesopores (2-50 nm), and macropores (> 50 nm). The porosity (together with the pore volume and pore size distribution) directly impacts a variety of other parameters, including density, mechanical stability, cation exchange capacity, and water retention capacity[33,34].
Functional attributes of activated carbon
Activated carbons are available in various forms and have unique surface properties. The physical and chemical surface attributes of AC, such as surface area, pore size, pore volume, and functional groups, are modified to provide different surface qualities and removal effectiveness. The precursor, activating agent, activation temperature, and production technique influence these characteristics. There are two ways to activate AC derived from carbonaceous sources: physically (thermally) or chemically[31,35,36]. Biosynthesis and mechanism of action of activated carbon, along with some notable advantages and limitations, are shown in Figure 5.
Figure 5. Biosynthesis and mechanism of action of activated carbon along with some notable advantages and limitations.
The biochar produced in pyrolysis is utilized to make activated carbon as a byproduct by combining reactive gasses (CO2) and steam with dehydrating or oxidizing substances (KOH, ZnCl2 and H3PO4) or dehydrating or oxidizing agents (KOH). The presence of naturally occurring heteroatom doping, such as that of oxygen, nitrogen, and sulfur, improves surface wettability and serves as an electroactive site[31]. Acidic and basic functional groups impact AC surface charge and, consequently, its adsorption characteristics. The AC typically has a hydrophobic surface, but when the AC surface has more functional groups that include oxygen adsorption, organic compounds are reduced due to the preference for water molecules over organic molecules[35]. Depending on the application, the characteristics of activated carbon can be adjusted, ranging from high porosity to high conductivity[31]. Other notable characteristics and suitability of activated carbon for remediation applications are shown in Figure 6.
Adsorptive removal of antibiotics
Graphene-assisted removal of antibiotics
Graphene is a great adsorbent candidate, which consists of a two-dimensional (2D) sheet made of a single layer of sp2 hybridized carbon atoms arranged in a honeycomb pattern that allows all atoms to be exposed and, therefore, easily contactable. Due to their porous structure and large surface area, antibiotics’ diffusion or surface reaction is possible using graphene adsorbents to achieve rapid and efficient adsorption[37]. Thanks to its unique composition traits, it has high sensitivity in sensor design, even down to the single molecule, which is utilized to adsorb aromatic organic components via π-π electron coupling or van der Waals interactions[38].
The adsorption process is intimately connected to the graphene/GBNPs’ surface characteristics, particularly concerning the surface area and pore structure. The attributes of antibiotics, as well as surface functional groups, may have an impact on the adsorption process. π-π interactions, hydrogen bonds, and electrostatic interactions are the major antibiotic adsorption interactions with graphene/GBNPs, where π-π would be the most prominent overall[39]. The pore structure and surface area both significantly impact the adsorption of the antibiotics on graphene/GBNPs because they can alter the adsorption capacity. The area of active sites exposed to antibiotics develops with the surface area during adsorption[40].
The numerous π electrons in the graphene/GBNPs structure enable π-π interactions. The term “π-π interactions” refers to a subtype of van der Waals interactions that have been formed between π systems that contain unsaturated (poly)cyclic molecules[41]. They form due to an attraction between the positively charged molecule next to them and any conjugated system’s π negatively charged electron cloud. Other factors to consider are kinetics and isotherm. Where the adsorption kinetics allows knowing the adsorption mechanism, which in the case of antibiotics removal is the PSO model as it considers that chemisorption is the potential rate controlling stages involving covalence force through a sharing or exchange of electrons between the adsorbate and adsorbent[42]. On the other hand, the adsorption isotherm determines the effectiveness of adsorbents for the adsorption of antibiotics and describes the surface characteristics. In this case, two equations describe the adsorption better than other models, namely the Langmuir and Freundlich models. The Langmuir isotherm posits a monolayer adsorption with uniform binding sites, the same energy, and no transmigration between adsorbed species that takes place on a homogenous surface during the absorption of antibiotic compounds[38]. In contrast, the Freundlich isotherm is an empirical equation that assumes multilayer adsorption onto heterogeneous surfaces to how the adsorbate is adsorbing to the adsorbent surface[43].
The surface hydrophobicity and ease of agglomeration in aqueous solutions of graphene serve as its primary drawbacks for usage as an adsorption mechanism in practical applications. Therefore, functionalized graphene must be generated to address these issues[44]. As an excellent option, graphene oxide (GO) provides graphene with vast quantities of oxygen atoms on the surface of the resulting composite as hydroxyl, epoxy, and carboxyl groups. The synthesis of this material is always produced by the intense oxidation of graphite, as a precursor for the synthesis of graphene, via a modified Hummer Method, which makes it easily producible compared to other nanomaterials[45]. Most antibiotics properly interact with GOs through π-π stacking. All these functional groups on the GOs make it remarkably hydrophilic due to its oxygen atoms and delocalized conjugated electrons on its surface[46]. Thereby enabling its excellent adsorption capacities and great dispersibility as a significant promise for the simple preconcentration of several types of water pollutants[47].
For example, Gao et al.[47] look into the use of GOs for adsorption and removal of tetracycline (TC) from aqueous environments and found that it has a strong affinity with the surface of GO via π-π interactions and cation-π bonding. Where the hexagonal cells of graphene oxide and the tetracycline molecule’s ring structure enable π-π interactions between them, and cation-π bonding is most likely to occur between the amino group on the ring C4 of tetracycline and the π-electron-rich structures of graphene. This led to a maximum theoretical adsorption capacity of 313 mg g-1 calculated by the Langmuir model displaying a maximum adsorption capacity at 231 nm due to the π-π interactions of aromatic C = C bonds. In other cases, Jia et al.[48] investigated the removal of three different sulfonamides (sulfadiazine, sulfamethoxazole, and sulfadoxine) using GO in combination with MIL-101(Cr), a metal-organic framework (MOF) that exhibits excellent chemical stability in addition to being reasonably priced. Due to its low atom density in its structures, this MOF has the disadvantage of having limited dispersive forces to bind small molecules. Because of this, the authors decided to utilize the qualities of GO to create a MIL-101(Cr)@GO compound that would enhance the dispersive forces and raise the porosity of the materials. This effort produced a material with a surface area of 3351.64 m2 g-1 and a maximum adsorption capacity of 135.14, 101.01, and 137.14 mg g-1 for sulfadiazine, sulfamethoxazole, and sulfadoxine, respectively. As for other antibiotics, metronidazole (MNZ) and trimethoprim (TMP) have been reported for been adsorbed by GO at 298 K. There, predominantly due to π-π dispersive interactions, the GO displayed its highest adsorption uptakes for TMP at pH = 10 and MNZ at pH = 7 of 218 mg/g and 190 mg/g, respectively[49]. Çalışkan Salihi et al.[50] found that the maximum adsorption capacity of TMP in 1 h is 204.08 mg/g. Also found that the adsorption process of TMP suggests intense competition for substrate sites from solvent or other adsorbed species molecules. It is also worth mentioning that they studied the adsorption capacity of isoniazid (INH), which is moderate as it only manages to adsorb 13.89 mg/g, because of a lack of competition with the solvent and mainly electrostatic attractions with the help of π-π dispersion interactions.
Because of the strong van de Waals interaction between the graphene sheets, 2D graphene is prone to aggregate, which reduces the surface area and impairs the efficient use of its exceptional properties[51]. Researchers have created new materials to address these problems, as Yao et al.[52] prepared a 3D porous network structure with the mixture of cellulose nanofibers (CNF) and GO by using a one-step ultrasonication method. This process resulted in an interconnected planar ultrathin network sheet in which the GOs nanosheets covered the CNF allowing the free diffusion and interaction of antibiotics into the CNF/GO aerogel’s interconnected 3D network structure by three different material properties of the structural network. Firstly, this could be via electrostatic attraction by facilitating the free diffusion of the antibiotics and ensuring mass transport to the internal 3D cross-linking structure. Secondly, with a focus on π-π stacking, CNF/GO may function as an electron acceptor, which is useful for adsorbing antibiotics with conjugate or unsaturated double-bond structures. Lastly, several hydroxyl groups allow the creation of hydrogen bonds with antibiotic molecules. With all of this, the team manages to evaluate six different categories of antibiotics which exhibit the following maximum theoretical adsorption capacity corresponding to the Langmuir model: 454.6 mg/g for tetracyclines, 128.3 mg/g for quinolones, 227.3 mg/g for sulfonamides, 418.7 mg/g for chloramphenicol, 230.7 mg/g for β-Lactams and 291.8 mg/g for macrolides.
Finally, as for the main factors that influence this adsorption, it was discovered that the initial concentration, pH, temperature, and contact time are vital to consider when looking for the optimal adsorption capacity. It was found that for tetracycline, doxycycline (DCN), and ciprofloxacin (CIP), an initial pH range of 6 to 7 and a contact period of 100 to 200 minutes are ideal, while a temperature increase from 25 °C to 45 °C resulted in a decrease in the monolayer adsorption result[46].
Biochar-assisted removal of antibiotics
Biochar is the carbonaceous solid product resulting from the pyrolysis of biomass materials[53]. Its surface features and unique qualities, such as its high cation exchange capacity (CEC), large specific surface area, abundance of functional groups that contain oxygen, and high mineral content, allow it to be utilized as an efficient adsorbent[54]. This, including the fact that it is considered a low-cost material, has attracted the interest of different researchers to use it as an effective absorption mechanism for antibiotics in aquatic environments. However, according to studies, the primary adsorption processes may involve chemical adsorption, including hydrogen bond and π-π electron-donor-acceptor (EDA) interactions. Therefore, increasing the number of π-π EDA contacts on the surface of biochar might be useful in boosting adsorption effectiveness. Therefore, for it to be truly efficient, it needs to be modified in order to overcome disadvantages such as low density, small particle size, and difficulty in splitting from water[55]. Currently, metal oxide and metal salt modification, clay mineral modification, acid-base modification, and ball milling modification are some techniques often employed to enhance the adsorption ability of biochar.
The biochar shows high electrostatic attraction, precipitation, and anion exchange properties after being modified with metal oxide and metal salts[56]. According to studies, the distribution of sulfurized nanoscale zero-valent iron (nZVI) to phosphorus-functionalized biochar (pBCS-nZVI) may improve the removal of florfenicol by a 4.3-fold[57]. For example, Cu-biochar was examined for the adsorption of doxycycline hydrochloride and demonstrated an adsorption capability of 93.22%[58]. Another example created via a hydrothermal method is a Fe-Cu layered double hydroxide mix with biochar that exhibited a 97.6% adsorption of cefazolin sodium in a water solution[59].
As for the clay mineral modification process, there are two different pathways to approach the modification. The first one includes mixing the clay minerals with clay minerals for pyrolysis, while the other method uses the resulting pyrolyzed biochar and then mixes it with clay minerals. With these conditions, it has been noticed that biochar enhances its porosity as it also improves its interface compatibility[60]. Ashiq et al.[61] examined the ciprofloxacin adsorption onto biochar formed from municipal solid waste and a composite material created by mixing its biochar with bentonite clay. As determined by isotherm modeling, the greatest amount of ciprofloxacin that the composite could absorb was 190 mg/g, which was almost 40% more than what could have been absorbed by the pure MSW-BC. Furthermore, another environmentally friendly adsorbent was created as a composite from montmorillonite based on corncob biochar to adsorb atenolol resulting in a composite capable of attaining a maximum equilibrium capacity of 86.86 mg/g[60].
The most popular modification method is using acid or alkali reactants. This technique alters the physical adsorption by changing the surface properties of the adsorbent, specific surface area, and pore structure of the biochars by largely increasing them. Additionally, the C-OH and C-H functional groups created by acid-base alteration also play a significant part in the chemical adsorption process, changing the biochars’ ability to adsorb substances[62]. Modified sludge biochar containing iron/zinc (Fe/Zn), phosphoric acid (H3PO4), or both (Fe/Zn + H3PO4) were generated and tested for their ability to adsorb fluoroquinolone antibiotics such as ciprofloxacin, norfloxacin (NOR), and ofloxacin (OFL) from water. The results indicated that the surface area, total pore volume, mesoporous volume, pore diameter (Dp), and oxygen-containing functional groups increased in the combination of Fe/Zn + H3PO4-SBC. In addition, CIP, NOR, and OFL demonstrated exceptional adsorption behavior, with maximum adsorption amounts of 83.7, 39.3, and 25.4 mg/g, respectively[63].
Finally, a technology not so investigated but promising for its sustainability and low cost is the ball milling of biochar. Biochar can be broken into a nanometer-sized powder using a ball mill[64]. During the grinding process, the grinding media can provide biochar with additional functional groups and expand its pore size to increase its ability to adsorb chemicals[65]. Huang et al[66] produced pyrolyzed biochar derived from bamboo and hickory chips at 450 °C to introduce 1.8 g of each sample to an agate jar with a 100:1 rate of grinding balls/biochar. This process reaches an adsorption efficiency of 83.3% and 89.6% while receiving a Langmuir maximum adsorption of 100.3 mg/g and 57.9 mg/g for sulfamethoxazole and sulfapyridine, respectively, for both results. As for tetracycline hydrochloride, another experiment showed potential for remediation in an aqueous solution as a ball-milled wheat stalk biochar pyrolyzed at 600 °C gave an adsorption amount of 84.5 mg/g[67].
CNTs-assisted removal of antibiotics
The carbon nanotubes, as it was previously mentioned, are single or multilayer materials based on graphene sheets rolled-up tubular that are used for the removal of antibiotics due to the electrical, mechanical, and thermal properties such as high melting point, thermal conductivity, and others[68]. Between the two types of CNTs, the single-wall carbon nanotubes represent a more efficient way to remove some substance, such as tetracycline, compared to other carbon-based materials[69] Yu et al.[70] reports the adsorption of ciprofloxacin by multi-walled carbon nanotubes. This antibiotic presents an antibacterial effect normally used for anthrax treatment and is not easily biodegradable. This carbon-based material was oxidized with different sodium hypochlorite concentrations to be loaded with oxygen concentrations. After several experiments, the maximum adsorption capacity was obtained with increasing oxygen capacity. Mousavi and Janjani[71] describe the application of the CNTs for the adsorption of Metronidazole, an antibiotic used for skin, vaginal, lung and other infections. These authors report the application and research in various papers of MWCNTs at controlled pH, temperature and contact time conditions, using an adsorbent dosage of 0.1 g/L. As a result, it is concluded that higher adsorption rates, higher adsorption per unit area[71].
Thermodynamics studies were developed to understand these carbon-based materials and their adsorption efficiency. Azarpira et al.[72] report Metronidazole adsorption (MNZ) using a Multi-Walled Carbon Nanotube. As a result, the adsorption increases when the adsorbent dosage increases, and it was established that this phenomenon presents a spontaneous nature. Shaban et al.[73] report Meropenem antibiotics’ adsorption and other similar compounds using MWCNTs. These authors evaluate the viability of removing these contaminants from water under laboratory conditions. However, more studies should be done around it for a better understanding. Gaálová et al.[74] implement a different approach by using and modifying single-walled carbon nanotube membranes for the elimination of Tetracycline 98.0% (antibiotic for bacterial infection), sulfamethoxazole or SM and trimethoprim or TMP (combination antibiotic in a 5:1 ratio used to treat ear infections, urinary tract infections and others). For the membrane preparation, 50 mg of unmodified SWCNTs was required that was dispersed with dimethylformamide (DMF). Later it was subjected to a size reduction process and vacuum filtration, obtaining a membrane that needed to be dried for 24 h. Hence, hydrophilic and modified hydrophobic SWCNTs were obtained, achieving a filtration of 98.8% of SM, 95.5% of TMP, and 87.0% of TC. Additionally, it was observed that thicker membranes present higher adsorption capacity and that mild oxidation increases the hydrophilicity and reactivity with the antibiotics[74].
Activated carbon-assisted removal of antibiotics
Given the material’s characteristics and its multiple sources of obtention, activated carbon is excellent for removing multiple drugs due to the porosity, commercial availability, and the high surface area, as previously mentioned[69]. Therefore, this material has been reported considerably in the literature to evaluate its impact and application. For that reason, different types of AC have been reported in the literature for the adsorption of various pharmaceutical compounds, such as tetracycline, a bactericidal that is not completely metabolized for any animal organism, so a certain part is discarded into the environment and becomes a residue that also impacts in the microorganisms that present an antibiotic resistance[75]. Martins et al.[75] report the removal from the water of this drug by NaOH-activated carbon. This material is generated based on macadamia nut shells pretreated to eliminate residues. A reactor and a heater were required to prepare the material at 500 °C to obtain the carbonized material that needed to be activated and mixed with NaOH (3:1 ratio), followed by other operations with different parameters. As a result, the activated carbon material developed is composed of 78% micropores. After performing adsorption tests, a yield of 19.79% was obtained with a surface area of 1524 m2/g. The maximum adsorption capacity for TC was around 453 mg/g[75].
Marzbali et al.[76] developed H3PO4-activated carbon material to adsorb TC. These were generated based on apricot stone or apricot nut shells as raw material using phosphoric acid (1:1 ratio) to activate it. The material presents a yield of 26.16%, a surface area of 307.6 m2/g, and a removal capacity of 308.33 mg/g. Additionally, these authors report that, based on thermodynamics, the adsorption process is endothermic and spontaneous. The activated carbon’s adsorption method represents a better alternative, given its non-toxicity and high removal potential. It is also established that the pH of the solution is directly related to the adsorption capacity[76].
Acosta et al.[77] also report the adsorption of Tc through activated carbon or tyre pyrolysis char (TPC) produced by potassium hydroxide (KOH) activation. In order to develop this Ac, tires were used as raw material. These tires represent a global environmental problem as considerable annual production and recycling measures remain challenging. Chemical activation by pyrolysis using KOH was required to synthesize it using a temperature rate of 600-800°C. As a result, maximum adsorption of 312 mg/g was obtained, which is a higher value than that reported for commercially activated carbon. Besides, this represents a good alternative that, in addition to reducing the presence of a contaminant in water, also improves the recycling of an important desire[77]. Furthermore, Ahmed outlines the adsorption of quinolone (one of the most important synthetic antibiotics used nowadays for humans and animals), tetracycline (used for the treatment of bacterial infections), and penicillin (broad spectrum bactericide) antibiotics from aqueous solution using Ac[78]. The Ac was synthesized by chemical and physical methods for its activation of it. Consequently, maximum adsorption capacities of 1340.8, 638.6, and 570.4 mg/g were reported for tetracyclines, quinolones, and penicillins, respectively[78].
For the removal of azithromycin (AZM) antibiotic, Balarak et al.[79] report the use of activated porous carbon. This pharmaceutical compound is used for bacterial infections and for the treatment of sexually transmitted diseases such as chlamidya and gonorrhea. Azolla filiculoides (AF) were required to synthesize this carbon-based material. The AF was treated by washing and drying, which would later be subjected to a size reduction process (milling). Then it was immersed in a ZnCl2 aqueous solution for 12 h. Afterwards, pyrolysis was performed under a nitrogen atmosphere at 350°C to 600°C, generating the activated porous carbon. Therefore, a maximum adsorption capacity of 364 mg/g was obtained. Additionally, a surface area of 484.1 m2/g and a porosity of 53.4%. Finally, it was concluded that this material is an effective and low-cost alternative for removing various antibiotics in water[79].
In the same way, the use of multiple materials that improve the characteristics and adsorption properties has been recently explored. Al-Musawi et al.[80] report the use of powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles (PAC@Fe3O4-MN) for the adsorption of ciprofloxacin antibiotics. For the synthesis of this material. A co-precipitation method was required. Then, a magnetization process was followed by coating the PAC. The carbon-based material presents excellent morphological properties, high paramagnetic value, and valuable active groups. The experiment developed for the adsorption shows that this material was 100% efficient, with a maximum adsorption capacity of 109.833 mg/g[80]. Fu et al.[81] report the adsorption of six frequently quinolone antibiotics using carbonaceous materials such as powdered activated carbon. The antibiotics required for the study were norfloxacin, ciprofloxacin, ofloxacin, enrofloxacin, sarafloxacin hydrochloride, and lomefloxacin hydrochloride. These authors describe the performance, mechanisms and models that better suit the behavior, which is a more theoretical approach. For that, adsorption kinetics and isotherm experiments were developed. As a result, a pseudo-second-order equation better suited the adsorption, and the adsorption capacity was affected by the pH[81].
LEFTOVER CHALLENGES AND FUTURE PERSPECTIVES
Due to technical difficulties such as scale manufacturing, system setup, cost-effectiveness, market readiness issues, and laboratory development delays, only a small number of carbon materials are on the market[38]. Furthermore, material flaws may severely influence the removal performance of antibiotics, so new environmentally friendly processes must be created to manufacture high-quality materials at a low cost. The development of energy-efficient, cost-effective technologies for producing biochar and activated carbon at high volumes while using byproducts (bio-oil and gases) is also crucial to reach the full potential of this technology[32]. In addition, some materials, for example, CNTs, are more expensive than other nano-carbon materials, so it is necessary to bring down the cost to a reasonable level by finding new carbon sources that are affordable and abundant[30].
Using a single treatment method to remove pollutants is not the best action. Adsorption is only one example of a phase-change process that only temporarily solves the issue since it produces a concentrated phase[18]. Exploring how to incorporate different strategies to maximize the outcome is crucial. A good opportunity would be to incorporate catalytic properties that could break down antibiotics into innocuous residues. Real-water bodies are a complex mixture of molecules with different natures. Therefore, future studies should focus on understanding how other pollutants may interfere with the removal of antibiotics and the interaction between carbon materials, antibiotics, and other pollutants. There is still much to learn about carbon-based materials, and there is still room to develop and implement their usage in the contaminant removal sector.
CONCLUSIONS
Due to the overuse of antibiotics, the level of these pollutants in the environment has significantly risen above the environmental safety threshold. Therefore, four different carbon-based materials have been introduced and developed as to why their manufacture is important to remediate antibiotic-contaminated waters attributable to their affordable price, environmental friendliness, and excellent adsorption capacity. The adsorption of antibiotics by carbon-based materials was considerably affected by the properties of the same carbon-based materials, the physical and chemical features of antibiotics, and the chemistry of the background solution. The main mechanism of adsorption in each of the materials includes some π-π interaction, which still needs to be explored in different case studies. Considering the above critiques discussed with key examples under different sections and subsections, it is concluded that antibiotics’ environmental transportation fate and various modifications enhance the inherent adsorption capability of reviewed carbon-based materials. Also, it was seen that with time the scope of investigations is getting broader because of the excellent efficiency of adsorption exhibited and facile modification. Nevertheless, there are still further studies to be made to explore the potential interactions that may occur during a non-simulated test where there are more factors to consider, such as environment, concentrations, and reaction while dealing with multiple pollutants simultaneously.
DECLARATIONS
Authors’ contributionsConceptualization, data curation, Figures, writing - original draft preparation: Cruz-Cruz A
Conceptualization, data curation, Figures, writing - original draft preparation: Rivas-Sanchez A
Conceptualization, data curation, Table, writing - original draft preparation: Gallareta-Olivares G
Writing - original draft preparation, writing - review & editing: González-González RB
Writing - original draft preparation, writing - review & editing: Cárdenas-Alcaide MF
Conceptualization, Figures, Table, writing - original draft preparation, writing - review & editing, resources, supervision, project administration, funding acquisition: Iqbal HMN
Resources, writing - original draft preparation, writing - review & editing: Parra-Saldívar R
Availability of data and materialsNot applicable.
Financial support and sponsorshipConsejo Nacional de Ciencia y Tecnología (CONACyT) Mexico and Tecnologico de Monterrey, Mexico are thankfully acknowledged for supporting this work under Sistema Nacional de Investigadores (SNI) program awarded to Hafiz M.N. Iqbal (CVU: 735340).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. García J, García-Galán MJ, Day JW, et al. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: increasing removal with wetlands and reducing environmental impacts. Bioresour Technol 2020;307:123228.
2. Bilal M, Mehmood S, Rasheed T, Iqbal HM. Antibiotics traces in the aquatic environment: persistence and adverse environmental impact. Curr Opin Environ Sci Health 2020;13:68-74.
3. Storto D, Nara LBC, Kozusny-andreani DI, et al. Seasonal dynamics of microbial contamination and antibiotic resistance in the water at the Tietê Ecological Park, Brazil. Water Air Soil Pollut 2021:232.
4. Grenni P, Ancona V, Barra Caracciolo A. Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 2018;136:25-39.
5. Qaswar M, Yiren L, Jing H, et al. Soil nutrients and heavy metal availability under long-term combined application of swine manure and synthetic fertilizers in acidic paddy soil. J Soils Sediments 2020;20:2093-106.5.
6. Bilal M, Ashraf SS, Barceló D, Iqbal HMN. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci Total Environ 2019;691:1190-211.
7. Bilal M, Rizwan K, Adeel M, Barceló D, Awad YA, Iqbal HMN. Robust strategies to eliminate endocrine disruptive estrogens in water resources. Environ Pollut 2022;306:119373.7.
8. González-González RB, Sharma A, Parra-Saldívar R, Ramirez-Mendoza RA, Bilal M, Iqbal HMN. Decontamination of emerging pharmaceutical pollutants using carbon-dots as robust materials. J Hazard Mater 2022;423:127145.8.
9. Chaturvedi P, Shukla P, Giri BS, et al. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: a review on emerging contaminants. Environ Res 2021;194:110664.
10. Zuo R, Liu X, Zhang Q, et al. Sulfonamide antibiotics in groundwater and their migration in the vadose zone: a case in a drinking water resource. Ecol Eng 2021;162:106175.
11. Szekeres E, Chiriac CM, Baricz A, et al. Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas. Environ Pollut 2018;236:734-44.
12. PubChem. Available from: https://pubchem.ncbi.nlm.nih.gov/ [Last accessed on 9 Feb 2023].
13. Drugs.com. Available from: https://www.drugs.com/ [Last accessed on 9 Feb 2023].
14. DrugBank Online. Available from: https://go.drugbank.com/ [Last accessed on 9 Feb 2023].
15. Zhang X, Guo W, Ngo HH, Wen H, Li N, Wu W. Performance evaluation of powdered activated carbon for removing 28 types of antibiotics from water. J Environ Manage 2016;172:193-200.
16. Aguilar-pérez K, Avilés-castrillo J, Ruiz-pulido G. Nano-sorbent materials for pharmaceutical-based wastewater effluents - an overview. Case Stud Chem Environ Eng 2020;2:100028.
17. Aguilar-Pérez KM, Avilés-Castrillo JI, Ruiz-Pulido G, Medina DI, Parra-Saldivar R, Iqbal HMN. Nanoadsorbents in focus for the remediation of environmentally-related contaminants with rising toxicity concerns. Sci Total Environ 2021;779:146465.
18. Gopinath KP, Vo DN, Gnana Prakash D, Adithya Joseph A, Viswanathan S, Arun J. Environmental applications of carbon-based materials: a review. Environ Chem Lett 2021;19:557-82.
19. Araújo RG, González-González RB, Martinez-Ruiz M, et al. Expanding the scope of nanobiocatalysis and nanosensing: applications of nanomaterial constructs. ACS Omega 2022;7:32863-76.
20. Cruz-cruz A, Gallareta-olivares G, Rivas-sanchez A, et al. Recent advances in carbon dots based biocatalysts for degrading organic pollutants. Curr Pollution Rep 2022;8:384-94.20.
21. González-González RB, Parra-Saldívar R, Alsanie WF, Iqbal HMN. Nanohybrid catalysts with porous structures for environmental remediation through photocatalytic degradation of emerging pollutants. Environ Res 2022;214:113955.
22. González-González RB, Flores-Contreras EA, Parra-Saldívar R, Iqbal HMN. Bio-removal of emerging pollutants by advanced bioremediation techniques. Environ Res 2022;214:113936.
23. Rivas-Sanchez A, Cruz-Cruz A, Gallareta-Olivares G, González-González RB, Parra-Saldívar R, Iqbal HMN. Carbon-based nanocomposite materials with multifunctional attributes for environmental remediation of emerging pollutants. Chemosphere 2022;303:135054.
24. Rodríguez-hernández JA, Araújo RG, López-pacheco IY, et al. Environmental persistence, detection, and mitigation of endocrine disrupting contaminants in wastewater treatment plants - a review with a focus on tertiary treatment technologies. Environ Sci Adv 2022;1:680-704.
25. Mashkoor F, Nasar A, Inamuddin. Carbon nanotube-based adsorbents for the removal of dyes from waters: a review. Environ Chem Lett 2020;18:605-29.
26. Bhuyan MSA, Uddin MN, Islam MM, Bipasha FA, Hossain SS. Synthesis of graphene. Int Nano Lett 2016;6:65-83.
27. Papageorgiou DG, Kinloch IA, Young RJ. Mechanical properties of graphene and graphene-based nanocomposites. Prog Mater Sci 2017;90:75-127.
28. Akinwande D, Brennan CJ, Bunch JS, et al. A review on mechanics and mechanical properties of 2D materials - Graphene and beyond. Extreme Mech Lett 2017;13:42-77.
29. Gupta N, Gupta SM, Sharma SK. Carbon nanotubes: synthesis, properties and engineering applications. Carbon Lett 2019;29:419-47.
30. Rahman G, Najaf Z, Mehmood A, et al. An overview of the recent progress in the synthesis and applications of carbon nanotubes. C J Carbon Res 2019;5:3.
31. Kar KK. Handbook of nanocomposite supercapacitor materials II. Cham: Springer International Publishing; 2020.
32. Cha JS, Park SH, Jung S, et al. Production and utilization of biochar: a review. J Ind Eng Chem 2016;40:1-15.
34. Leng L, Xiong Q, Yang L, et al. An overview on engineering the surface area and porosity of biochar. Sci Total Environ 2021;763:144204.
35. Mansour F, Al-hindi M, Yahfoufi R, Ayoub GM, Ahmad MN. The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: a review. Rev Environ Sci Biotechnol 2018;17:109-45.
36. Lesaoana M, Mlaba R, Mtunzi F, Klink M, Ejidike P, Pakade V. Influence of inorganic acid modification on Cr(VI) adsorption performance and the physicochemical properties of activated carbon. S Afr J Chem Eng 2019;28:8-18.
37. Ji L, Chen W, Xu Z, Zheng S, Zhu D. Graphene nanosheets and graphite oxide as promising adsorbents for removal of organic contaminants from aqueous solution. J Environ Qual 2013;42:191-8.
38. Li MF, Liu YG, Zeng GM, Liu N, Liu SB. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: a review. Chemosphere 2019;226:360-80.
39. Moradi SE. Highly efficient removal of amoxicillin from water by magnetic graphene oxide adsorbent. Available from: http://chemicalbulletin.upt.ro/admin/articole/72848art_3(41-48).pdf [Last accessed on 9 Feb 2023].
40. Yusuf M, Elfghi FM, Zaidi SA, Abdullah EC, Khan MA. Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv 2015;5:50392-420.
41. Jian M, Xie H, Xia K, Zhang Y. Challenge and opportunities of carbon nanotubes. Industrial applications of carbon nanotubes. Elsevier; 2017. pp. 433-76.
42. Song W, Yang T, Wang X, et al. Experimental and theoretical evidence for competitive interactions of tetracycline and sulfamethazine with reduced graphene oxides. Environ Sci Nano 2016;3:1318-26.
43. Zhang Y, Jiao Z, Hu Y, et al. Removal of tetracycline and oxytetracycline from water by magnetic Fe3O4@graphene. Environ Sci Pollut Res Int 2017;24:2987-95.
44. Bai L, Li Z, Zhang Y, et al. Synthesis of water-dispersible graphene-modified magnetic polypyrrole nanocomposite and its ability to efficiently adsorb methylene blue from aqueous solution. Chem Eng J 2015;279:757-66.
45. Yao N, Li C, Yu J, et al. Insight into adsorption of combined antibiotic-heavy metal contaminants on graphene oxide in water. Sep Purif Technol 2020;236:116278.
46. Rostamian R, Behnejad H. A comprehensive adsorption study and modeling of antibiotics as a pharmaceutical waste by graphene oxide nanosheets. Ecotoxicol Environ Saf 2018;147:117-23.
47. Gao Y, Li Y, Zhang L, et al. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J Colloid Interf Sci 2012;368:540-6.
48. Jia X, Li S, Wang Y, Wang T, Hou X. Adsorption behavior and mechanism of sulfonamide antibiotics in aqueous solution on a novel MIL-101(Cr)@GO composite. J Chem Eng Data 2019;64:1265-74.48.
49. Carrales-alvarado D, Rodríguez-ramos I, Leyva-ramos R, Mendoza-mendoza E, Villela-martínez D. Effect of surface area and physical-chemical properties of graphite and graphene-based materials on their adsorption capacity towards metronidazole and trimethoprim antibiotics in aqueous solution. Chem Eng J 2020;402:126155.
50. Salihi E, Wang J, Kabacaoğlu G, Kırkulak S, Šiller L. Graphene oxide as a new generation adsorbent for the removal of antibiotics from waters. Sep Sci Technol 2021;56:453-61.
51. Liu Y, Huang S, Zhao X, Zhang Y. Fabrication of three-dimensional porous β-cyclodextrin/chitosan functionalized graphene oxide hydrogel for methylene blue removal from aqueous solution. Colloids Surf A Physicochem Eng Asp 2018;539:1-10.
52. Yao Q, Fan B, Xiong Y, Jin C, Sun Q, Sheng C. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci Rep 2017;7:45914.
53. Vithanage M, Mayakaduwa SS, Herath I, Ok YS, Mohan D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016;150:781-9.
54. Zhang Y, Cao B, Zhao L, et al. Biochar-supported reduced graphene oxide composite for adsorption and coadsorption of atrazine and lead ions. Appl Surf Sci 2018;427:147-55.
55. Zhang A, Li X, Xing J, Xu G. Adsorption of potentially toxic elements in water by modified biochar: a review. J Environ Chem Eng 2020;8:104196.
56. Wu L, Wei C, Zhang S, Wang Y, Kuzyakov Y, Ding X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J Clean Prod 2019;235:901-9.
57. Xu J, Cao Z, Wang Y, et al. Distributing sulfidized nanoscale zerovalent iron onto phosphorus-functionalized biochar for enhanced removal of antibiotic florfenicol. Chem Eng J 2019;359:713-22.
58. Liu S, Xu WH, Liu YG, et al. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Sci Total Environ 2017;592:546-53.
59. Gholami P, Dinpazhoh L, Khataee A, Hassani A, Bhatnagar A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J Hazard Mater 2020;381:120742.
60. Fu C, Zhang H, Xia M, Lei W, Wang F. The single/co-adsorption characteristics and microscopic adsorption mechanism of biochar-montmorillonite composite adsorbent for pharmaceutical emerging organic contaminant atenolol and lead ions. Ecotoxicol Environ Saf 2020;187:109763.
61. Ashiq A, Sarkar B, Adassooriya N, et al. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019;236:124384.
62. Hu X, Xue Y, Long L, Zhang K. Characteristics and batch experiments of acid- and alkali-modified corncob biomass for nitrate removal from aqueous solution. Environ Sci Pollut Res Int 2018;25:19932-40.
63. Ma Y, Li P, Yang L, et al. Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal. Ecotoxicol Environ Saf 2020;196:110550.
64. Wang B, Gao B, Fang J. Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol 2017;47:2158-207.
65. Qin Y, Zhu X, Su Q, et al. Enhanced removal of ammonium from water by ball-milled biochar. Environ Geochem Health 2020;42:1579-87.
66. Huang J, Zimmerman AR, Chen H, Gao B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ Pollut 2020;258:113809.
67. Xiang W, Wan Y, Zhang X, et al. Adsorption of tetracycline hydrochloride onto ball-milled biochar: governing factors and mechanisms. Chemosphere 2020;255:127057.
68. Sabzehmeidani MM, Mahnaee S, Ghaedi M, Heidari H, Roy VAL. Carbon based materials: a review of adsorbents for inorganic and organic compounds. Mater Adv 2021;2:598-627.
69. Nazal MK. An overview of carbon-based materials for the removal of pharmaceutical active compounds. In: Bartoli M, Frediani M, Rosi L, editors. Carbon-based material for environmental protection and remediation. IntechOpen; 2020.
70. Yu F, Sun S, Han S, Zheng J, Ma J. Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem Eng J 2016;285:588-95.
71. Mousavi SA, Janjani H. Antibiotics adsorption from aqueous solutions using carbon nanotubes: a systematic review. Toxin Reviews 2020;39:87-98.
72. Azarpira H, Mahdavi Y, Khaleghi O, Balarak D. Thermodynamic studies on the removal of metronidazole antibiotic by multi-walled carbon nanotubes. Available from: https://www.scholarsresearchlibrary.com/articles/thermodynamic-studies-on-the-removal-of-metronidazole-antibiotic-by-multiwalled-carbon-nanotubes.pdf [Last accessed on 9 Feb 2023].
73. Shaban MAA, Ibrahim MA, M-ridha MJ, Hussein HA. Adsorption of meropenem antibiotics from aqueous solutions on multi-walled carbon nanotube. Int Rev Civ Eng 2020;11:283-93.
74. Gaálová J, Bourassi M, Soukup K, et al. Modified single-walled carbon nanotube membranes for the elimination of antibiotics from water. Membranes 2021;11:720.
75. Martins AC, Pezoti O, Cazetta AL, et al. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem Eng J 2015;260:291-9.
76. Marzbali MH, Esmaieli M, Abolghasemi H, Marzbali MH. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: a batch study. Process Saf Environ Prot 2016;102:700-9.
77. Acosta R, Fierro V, Martinez de Yuso A, Nabarlatz D, Celzard A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016;149:168-76.
78. Ahmed MJ. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons. Environ Toxicol Pharmacol 2017;50:1-10.
79. Balarak D, Mahvi AH, Shahbaksh S, Wahab MA, Abdala A. Adsorptive removal of azithromycin antibiotic from aqueous solution by azolla filiculoides-based activated porous carbon. Nanomaterials 2021;11:3281.
80. Al-musawi TJ, Mahvi AH, Khatibi AD, Balarak D. Effective adsorption of ciprofloxacin antibiotic using powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles. J Porous Mater 2021;28:835-52.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].