Is it the appropriate syringe filter? The loss of PPCPs during filtration by syringe filter
Abstract
Syringe filters are used to separate solids from liquids before chromatography analysis for the removal of particulate matter to avoid column blockage. The inappropriate selection of syringe filters may lead to the interception of micropollutants in samples (especially aqueous phase samples) and inaccurate quantification. In this study, mass losses of typical micropollutants - pharmaceutical and personal care products (PPCPs) - by syringe filters were evaluated considering the material of syringe filters, the pore size of syringe filters, solvents, and pre-rinsing. The lowest mass losses of 57 PPCPs were observed by hydrophobic- polytetrafluoroethylene (PTFE) (median value was 10%), but for quinolone (7-37%) and macrolide antibiotics (9-52%), the mass losses were still considerable. By changing the pore sizes of filters, the interception of quinolone and macrolide antibiotics by hydrophobic-PTFE was not improved. In contrast, by increasing the proportion of methanol in the solvent and discarding the first 1 mL pre-rinsing samples, the mass losses of quinolone and macrolide antibiotics by hydrophobic-PTFE can be considerably decreased. This study provides guidance for selecting appropriate filters for micropollutants before chromatography analysis of samples to guarantee the accuracy of the results.
Keywords
INTRODUCTION
Pharmaceutical and personal care products (PPCPs) comprise thousands of organic chemicals, including various human and veterinary drugs, personal care products, and household chemicals commonly used in daily life[1]. They have aroused widespread concern over the last decades[2], and they are considered emerging contaminants because they are frequently detected in the water cycle, including surface water, seawater, groundwater, and tap water[3-9], and may threaten the aquatic ecosystem and human health[10,11]. For instance, although concentrations of PPCPs are generally at the nanogram per liter level in surface waters, they can induce deleterious effects, such as endocrine disruption, inhibition of primary productivity, and other adverse effects[12]. There is an emerging interest in their fate in the environment and their removal from the aqueous phase. Until now, various experimental works have been carried out to study their environmental behavior and eliminate their concentrations in the water environment in laboratory studies. Usually, liquid chromatography (LC) or coupled with mass spectrometry (LC-MS) is employed for the analysis of PPCPs. Before being injected into the analytical instrument, samples should be filtered to avoid column blockage.
Syringe filters are commonly used in the field and laboratory, given their ease of use and availability[13,14]. However, several studies have demonstrated the caveats to drugs filtration using the filters[15-19]. A fundamental issue is the potential adsorption of investigated PPCPs by filters during filtration. The loss (defined as the decreased concentration of investigated compounds after filtration in the aqueous phase) of PPCP analytes may greatly distort the results. However, this problem may not be realized in some studies, and details about the filters adopted are not always described[20-23].
Most microfilters are made from synthetic organic polymers, which may interact with organic chemicals. For instance, polyvinyl chloride was shown to sorb organic chemicals such as chlorinated benzenes[24], polyethylene sorbs trichloroethylene and atrazine[25], and poly(isobutyl methacrylate) sorbs phenanthrene[26]. The losses of perfluoroalkyl carboxylates and perfluoroalkyl sulfonates were up to 15%[27] and 30%[28] when 0.45 μm glass fiber filters were used, 38%[27] and 60%[28] when 0.45 μm nylon filters were employed, and 98% when 0.45 μm polytetrafluoroethylene (PTFE) filters were adopted[27]. Thus, it is important to select an appropriate filter, considering that various membranes used in filters have different chemical compatibility. However, there is a lack of systematic research to investigate the mass losses of PPCPs during syringe filtration and propose measures for reducing the losses during the filtration step.
In the present study, we evaluated the mass loss of 57 PPCPs with different physicochemical properties by syringe filters with different filter materials and filter pore sizes in three matrix solutions. The objectives were to quantitatively determine the mass loss of emerging contaminants in experimental studies and provide useful suggestions on the selection of syringe filters in related experimental research.
EXPERIMENTAL
Chemicals and reagents
Fifty-seven PPCPs were selected [Supplementary Table 1] based on their extensive usage and application as well as their frequent detections in the environment[29]. These PPCPs can be classified into antibiotics [including sulfonamides (SAs), tetracyclines (TCs), quinolones (QLs), and macrolides (MAs)] and non-antibiotics (lipid regulators, non-steroidal anti-inflammatory drugs, etc.). They have numerous functional groups and different physicochemical properties, e.g., a broad polarity range (octanol-water partition coefficient, log Kow values from 0 to 4.79).
Filtration tests
Six types of 13 mm diameter syringe filters were selected [Table 1] in this study. In addition, three solutions, i.e., Milli-Q water (Millipore, Merck, Germany), 50:50 (v/v) methanol:water, and pure HPLC-grade methanol (Macklin, Shanghai), were tested with the purpose to reduce the PPCP mass loss by changing the solvent phase.
Specifications of the selected filters
| Membrane type | Abbreviation | Properties | Pore size (μm) |
| Polytetrafluoroethylene | PTFE | Hydrophilic | 0.22 |
| 0.45 | |||
| Hydrophobic | 0.22 | ||
| 0.45 | |||
| Nylon | NYL | Hydrophobic | 0.22 |
| 0.45 |
In the filtration test, the matrix solution was spiked with a mixture of 57 PPCP standards and the final PPCP concentrations were set at 100 μg/L. The prepared samples (1 mL) were then filtered by syringe filters [Table 1] and collected into 1.5 mL amber auto-injector glass vials. The syringe filters selected were produced by the same manufacturer to eliminate errors due to the differences among manufacturers. The mass loss of each PPCP was calculated according to the concentration differences for selected syringe filters. To ensure the reliability of the results, triplicate samples were filtrated for each syringe type in each matrix solution.
For each condition, duplicated samples (n = 3) were tested. Positive control samples (without filtration) spiked with PPCPs at the same concentration (n = 3) and blank samples (without the addition of target PPCPs) were also prepared in duplicate (n = 2). The measured concentrations in the positive blanks were used to correct the losses (e.g., sorption to container walls) before filtration[30-32], and blank samples were used to monitor the potential contamination during the experiments.
Sample preparation and analysis
Samples were spiked with a 50 μL internal standard (IS) mixture (1000 μg/mL of each individual IS, [Supplementary Table 2], vortexed for 1 min, and then analyzed using ultra-performance liquid chromatography (UPLC) system coupled to a triple quadrupole mass spectrometer (MS/MS) (Shimadzu, LCMS-8050, Japan), as described in[29]. A Shim-pack GIST-HP (G) C18 column (2.1 × 10 mm, 2 μm particle size) was connected with the analytical column (Shim-pack GIST C18 column, 2.1 × 100 mm, 2 μm particle size) to delay background peaks caused by solvent contamination and prevent interferences. All other instrumental parameters can be found in Supplementary Table 3.
Calculation of mass losses for PPCPs
Losses of individual PPCPs by syringe filters were calculated by comparing PPCP concentration obtained after filtration (Ci) with PPCP concentration without filtration (Ci, positive control) after subtracting the blank concentration (Ci, negative blank), as shown in Equation (1).
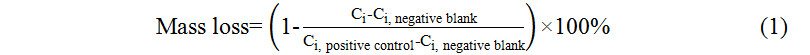
A mass loss larger than 20% was defined as considerable and therefore unacceptable for quantitative investigations[33]. The mass losses mentioned in this paper are median values.
Quality assurance and quality control
Quantification was based on linear regression calibration curves using an internal standard method. Calibration curves were constructed by plotting the concentrations of each analyte versus the ratios between the analyte peak area and the corresponding IS peak area using linear regression analysis[34]. The linearity and range of standard calibration curves (R2 = 0.995-0.999), the limit of detection, and the limit of quantity [Supplementary Table 4] were evaluated. In addition, statistical analysis was performed using the correlation analysis software (IBM SPSS Statistics 23, nonparametric tests, Mann-Whitney U-test).
RESULTS AND DISCUSSION
Impact of filter material
To investigate the performance of filters with different materials, Milli-Q water was employed as the solvent, considering that most studies about PPCP removal were conducted in Milli-Q water. The pore size of the filter was 0.22 μm, which is preferred for instrumental analyses, especially UPLC/MS/MS[35].
Figure 1 presents the mass losses of 57 PPCPs by filters with different materials in Milli-Q water. The mass losses between hydrophilic-PTFE filter and nylon filter were significantly different (nonparametric tests, Mann-Whitney U-test, P < 0.05). Although the median mass losses showed no significant differences, the mass losses by hydrophobic-PTFE filter were mainly in the range of 0-20%, while the ones by hydrophilic-PTFE filter and nylon filter were mainly concentrated in 0-20% and 90-100%, suggesting that the hydrophobic-PTFE filter was more suitable for the filtration of PPCPs in Milli-Q water. Hydrophobic-PTFE filters are commonly used in the field of chemical industry, textile industry, and water treatment due to several advantages, e.g., low analyte binding, good chemical compatibility, and solvent resistance[36]. The properties of low analyte binding may be related to the lower mass losses of most PPCPs by the hydrophobic-PTFE filter in our study.
Figure 1. Mass losses (%) of PPCPs in Milli-Q water after filtration using syringe filters made of three different materials. **Indicates that mass losses in the two types of syringe filters are significantly different by U-test at the 0.05 level; *indicates that the difference is not significant under the same condition. PPCP: Pharmaceutical and personal care products.
The mass loss performance of three filters varied for individual PPCPs [Figure 2A-C]. Note that the abbreviations of PPCPs are reported in Supplementary Table 1. For most SA and TC antibiotics, there was no obvious difference (i.e., mass loss of < 20%) by three filters [Figure 2A]. However, for QL and MA antibiotics, unexpectedly large mass losses were observed [Figure 2B]. Their mass losses followed the trend: hydrophilic-PTFE filter (up to 100%) > nylon filter (-6% to 98%) > hydrophobic-PTFE filter (7-52%). The authors of[37] investigated the interaction between filters and 43 widely detected basic, neutral, and acidic organic micropollutants in an aqueous solution, and found the majority of the compounds with higher log Kow values showed significant mass loss (up to 100%) to the tested materials. MA antibiotics in the present study were mainly hydrophobic (log Kow > 2) [Supplementary Table 1], and the mass losses of MA antibiotics by hydrophilic-PTFE filter could be related to the hydrophobic interactions. QL antibiotics investigated were hydrophilic (log Kow < 2), and the mass losses of QL antibiotics by the hydrophilic-PTFE filter were likely the result of hydrophilic modification. PTFE membranes without modification exhibited poor wettability due to hydrophobicity (internal chemical structure [-(-CF2-CF2-)n-])[38] and low surface energy. To facilitate the treatment of aqueous solutions, hydrophilic agents including amino, hydroxyl, carboxyl, and sulfonic acid groups were synthesized via hydrolytic polycondensation and free radical polymerization and steadily adhered to the surface of hydrophobic-PTFE membrane by a facile physical entanglement method[39]. These hydrophilic groups can generate hydrogen bonds in water, which were found to contribute to the retention and adsorption of endocrine-disrupting chemicals by membranes[40]. Therefore, the retention of QL antibiotics caused by the hydrogen bonds may be one of the reasons for their mass loss by a hydrophilic-PTFE filter. In addition, MA antibiotics contain hydroxyl or phenolic hydroxyl groups, which could be considered as an ideal proton donor for hydrogen bonds, promoting the formation of hydrogen bonds[40] and leading to enhanced adsorption of chemicals on a hydrophilic-PTFE filter. Therefore, the mass loss of MA antibiotics may be the result of the combined effect of hydrogen bonds and hydrophobicity. It should be mentioned that some PPCPs have a negative value of a mass loss, i.e., increased concentration after filtration. The concentration difference within ± 10% was probably due to the analytical errors. Only a few PPCPs exhibited a negative mass loss of > 10%, and the apparent higher concentration of analytes after filtration may be related to the co-elutes from the filters[35], which has an impact on the signal of PPCPs and their quantification.
Figure 2. Mass losses of PPCPs in Milli-Q water after filtration using three different types of syringe filters(A: TC and SA antibiotics; B: QLand MA antibiotics;
For the investigated non-antibiotics, the mass losses of most compounds (i.e., GLI, TOL, CF, CRO, BF, CBZ, WAR, THP, ACE, DF, and DEET) did not show a significant difference in three types of filters (within ± 20%) [Figure 2C]. However, for other non-antibiotics (i.e., SAL, SP, FBZ, ABZ, FLU, and GLY), larger mass losses were observed when hydrophilic-PTFE and nylon filters (87-100% and -3% to 100%, respectively) were used, while the hydrophobic-PTFE filter showed low adsorption (1-19%), except for FLU (34 %) and GLY (48%).
Impact of filter pore size
Normally, HPLC analysis requires sample filtration through filters with pore sizes of 0.45 or 0.22 μm before sample injection[35], and the impact of filter pore size on the losses of PPCPs in Milli-Q water was investigated.
Overall, the mass loss of PPCPs by nylon filters was barely affected by filter pore sizes. However, the adsorption of PPCPs by PTFE filters (especially hydrophilic ones) was sensitive to filter pore sizes (nonparametric tests, Mann-Whitney U-test, P < 0.05)-mass losses of PPCPs by the 0.22 μm filter were significantly higher than those by the 0.45 μm ones [Figure 3]. Multiple interactions, such as hydrogen bonding, electrostatic interactions, and hydrophobic interactions, are responsible for binding the analyte to the membrane surface[35]. Generally, microfiltration processes are primarily based on the principle of size exclusion, and solid removal is largely dependent on filter pore sizes[41]. However, as the molecular width and size of PPCPs were much smaller than the membrane pore sizes[42], the higher PPCP adsorption by the 0.22 μm PTFE filter might be the result of enhanced physical adsorption and/or enhanced electrostatic interaction, not size exclusion.
Figure 3. Mass losses (%) of PPCPs in Milli-Q water after filtration using different pore sizes of syringe filters. **Indicates that mass losses in the two types of syringe filters are significantly different by U-test at the 0.05 level; *indicates that the difference is not significant under the same condition. PPCP: Pharmaceutical and personal care products.
For individual PPCPs, the filter pore size has different influences. SAs and TCs still showed low PPCP mass loss (within ± 20%) by three filters and were barely affected by pore sizes (nonparametric tests, Mann-Whitney U-test, P > 0.05) [Figure 4A and B]. For QL antibiotics and non-antibiotics, the mass losses by the hydrophobic-PTFE and nylon filter were low (within ± 20%) and not sensitive to filter pore sizes [Figure 4C and D], while, for a hydrophilic-PTFE filter, the mass losses of QL antibiotics (nonparametric tests, Mann-Whitney U-test, P < 0.05) and non-antibiotics decreased (from 100% to 11% and from 8% to 4%, respectively) with the increasing of filter pore size. As the majority of these PPCPs were hydrophilic compounds, when they were dissolved in the aqueous phase, the interaction between PPCPs and hydrophilic-PTFE filter resulted in the building up of a “refuse layer”[43, 44] on membrane filters, leading to the greater interception of analytes. However, the increase in filter pore size cannot eliminate the mass losses of MA antibiotics regardless of the material of the filters [Figure 4E]. This result also supports the assumption that hydrophobic interaction and hydrogen bonding (not size exclusion) resulted in the mass losses of MA antibiotics by the hydrophilic-PTFE filter (as discussed in Section 3.1). Therefore, for MA antibiotics, further optimization should be conducted to reduce their loss during filtration.
Figure 4. Mass losses (%) of different PPCPs in Milli-Q water after filtration using syringe filters with different materials and pore sizes (A: TC antibiotics; B: SA antibiotics; C: QL antibiotics; D: non-antibiotics; E: MA antibiotics). **Indicates that mass losses in the two types of syringe filters are significantly different by U-test at the 0.05 level; *indicates that the difference is not significant under the same condition. PPCP: Pharmaceutical and personal care products.
Impact of matrix
Although a hydrophobic-PTFE filter with a pore size of 0.45 μm can realize a significant decrease in PPCP interception, it may also be inappropriate under certain conditions. For example, in many cases, UPLC (or UPLC-MS/MS) has been adopted to analyze PPCPs because it is more sensitive and faster than HPLC (or HPLC-MS/MS). However, the higher speed and higher peak capacity of UPLC also pose more stringent requirements on sample quality, e.g., the content of fine particles. Fine particles, which can go through the 0.45 μm filters, may result in column blockage. Thus, for UPLC-based analysis, it is recommended to use 0.22 μm filters. Therefore, tests were conducted to find whether the mass loss of PPCPs could be eliminated by changing the matrix solvent when 0.22 μm filters had to be employed.
A significant decrease in mass losses was observed for all three types of filters when the proportion of methanol in the solvent increased (nonparametric tests, Mann-Whitney U-test, P < 0.05) [Figure 5]. For the hydrophilic-PTFE filter, the median mass loss of PPCPs decreased from 11% (Milli-Q water) to 3% (50% methanol:water) and 3% (methanol), but for QL and MA antibiotics and non-antibiotics, the mass losses were still high (> 20%) [Supplementary Figure 1]. For the nylon filter, the mass losses of target PPCPs significantly decreased, and in methanol, the mass losses were the lowest for all the PPCPs except TCs. For the hydrophobic-PTFE filter, the increased proportion of methanol led to a further decrease in PPCP mass losses. The presence of organic solvents facilitates the partitioning of PPCPs in the solution phase with a simultaneous reduction of adsorption on filter membranes[41]. Therefore, a low loss (or in some cases no loss) of PPCPs can be achieved using hydrophobic-PTFE in pure methanol.
Figure 5. Mass losses (%) of PPCPs after filtration in different matrix systems. **Indicates that mass losses in the two types of syringe filters are significantly different by U-test at the 0.05 level; *indicates that the difference is not significant under the same condition. PPCP: Pharmaceutical and personal care products.
Impact of filter pre-rinsing
According to the section on the impact of a matrix, increasing the proportion of methanol can eliminate the mass losses of PPCPs to an acceptable level (within 20%). Nevertheless, most studies about PPCP removal are conducted in the aqueous phase. If the proportion of methanol in the samples needs to be increased before the analysis, the sample will be diluted, and the detection limits will be much higher, which is a big challenge for the experiments conducted at environmentally relevant concentration levels. Therefore, we conducted experiments to find out whether the interception of PPCPs in Milli-Q water could be eliminated by filter pre-rinsing when a hydrophobic-PTFE filter was employed [Figure 6].
Figure 6. Mass losses (%) of PPCPs in Milli-Q water on the hydrophobic-PTFE filter by pre-rinsing. **Indicates that mass losses in the two types of syringe filters are significantly different by U-test at the 0.05 level; *indicates that the difference is not significant under the same condition.
By discarding the first 1 mL pre-rinsing samples, decreased mass losses for target PPCPs were observed. Of these, the mass losses of TC and SA antibiotics and non-antibiotics [Figure 6] hardly affected (nonparametric tests, Mann-Whitney U-test, P > 0.05) by hydrophobic-PTFE filters can be eliminated to acceptable levels (within 20%) whether or not the first 1 mL of the filtered sample was discarded. After filtering a few milliliters of sample solution, the available active sites on the membrane for adsorption were occupied, and the amount of adsorbed PPCPs in the samples collected could be reduced. This is in agreement with previous studies[45]. For example, the authors of Ref.[37] indicated that, for loratadine compounds that are strongly affected by filter materials such as cellulose acetate, regenerated cellulose acetate, and polycarbonate, discarding a large amount of liquid (≥ 25 mL) before actual sampling can eliminate significant losses during filtration.
Conversely, although decreased mass losses for some QLs (from 7% to 6%) and MAs (31% to 19%) (nonparametric tests, Mann-Whitney U-test, P < 0.05) were observed on the hydrophobic-PTFE filter by pre-rinsing, individual compounds still showed considerable mass losses in Milli-Q water [Figure 6]. As a consequence, this method of pre-rinsing is not applied to all PPCPs. Future studies can be conducted to investigate the influence of discarded solution volume and to find out whether the performance could be improved by increasing the discarded solution volume. Nevertheless, if the amount of sample to be measured is not sufficient, then pre-rinsing is not an optimal choice.
CONCLUSIONS
The suitability of syringe filters for PPCP analysis in aqueous samples was evaluated considering the material of syringe filters, the pore size of syringe filters, solvents, and pre-rinsing. The results show that, for Milli-Q water samples, the lowest mass losses were observed by the hydrophobic-PTFE; however, the mass losses of quinolone and macrolide antibiotics were still considerably large (7-52%). By changing the pore sizes of filters, the interception of quinolone and macrolide antibiotics by hydrophobic-PTFE filter was not improved, while, by increasing the proportion of methanol in the solvent and discarding the first 1 mL pre-rinsing samples, the mass losses of quinolone and macrolide antibiotics by the hydrophobic-PTFE filter can be significantly decreased.
According to the obtained results, we recommend using a 0.22 μm hydrophobic-PTFE filter (with pre-rising) to filter a broad range of target PPCPs in most cases. If the target PPCPs are tetracyclines, sulfonamides, and non-antibiotics, i.e., GLI, TOL, CF, CRO, BF, CBZ, WAR, THP, ACE, DF, and DEET, 0.22 μm hydrophilic-PTFE, nylon, and hydrophobic-PTFE filter can be used. If macrolide antibiotics should be accurately determined, we recommend using a 0.22 μm hydrophobic-PTFE or nylon filter (with pre-rising), and the proportion of methanol in the solvent during the filtration should be increased. In addition, it should be mentioned that, for real water samples with different constituents from Milli-Q water, more efforts should be made to select suitable filters.
DECLARATIONS
Authors’ ContributionsReviewed literature, drafted the manuscript, had a leading role, served as the hub of communication among the authors: Dong WJ
Reviewed the manuscript and contributed to design of the study: Yu X, Wang JX
Checked and approved the final version for publication: Sui Q
Availability of data and materialsData can be published as supplementary information in the journal.
Financial support and sponsorshipThis research was partly supported by the National Natural Science Foundation of China (21777042 and 22076045), Shanghai Talent Development Funding (2020051), Shanghai Youth Talent Support Program, and the Fundamental Research Funds for the Central Universities.
Conflicts of interestAll author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Lin T, Yu S, Chen W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 2016;152:1-9.
2. Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 1999;107:907.
3. Heberer T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. Journal of Hydrology 2002;266:175-89.
4. Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 2002;131:5-17.
5. Hillebrand O, Nödler K, Licha T, Sauter M, Geyer T. Caffeine as an indicator for the quantification of untreated wastewater in karst systems. Water Res 2012;46:395-402.
6. Nödler K, Licha T, Fischer S, Wagner B, Sauter M. A case study on the correlation of micro-contaminants and potassium in the Leine River (Germany). Applied Geochemistry 2011;26:2172-80.
7. Nödler K, Licha T, Voutsa D. Twenty years later-atrazine concentrations in selected coastal waters of the Mediterranean and the Baltic Sea. Mar Pollut Bull 2013;70:112-8.
8. Reh R, Licha T, Geyer T, Nödler K, Sauter M. Occurrence and spatial distribution of organic micro-pollutants in a complex hydrogeological karst system during low flow and high flow periods, results of a two-year study. Sci Total Environ 2013;443:438-45.
9. Ternes T. The occurrence of micopollutants in the aquatic environment: a new challenge for water management. Water Sci Technol 2007;55:327-32.
10. Camacho-Muñoz D, Martín J, Santos JL, Aparicio I, Alonso E. Occurrence, temporal evolution and risk assessment of pharmaceutically active compounds in Doñana Park (Spain). J Hazard Mater 2010;183:602-8.
11. Deblonde T, Cossu-Leguille C, Hartemann P. Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health 2011;214:442-8.
12. Birch GF, Drage DS, Thompson K, Eaglesham G, Mueller JF. Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Mar Pollut Bull 2015;97:56-66.
13. Martinelango PK, Gümüş G, Dasgupta PK. Matrix interference free determination of perchlorate in urine by ion association-ion chromatography-mass spectrometry. Anal Chim Acta 2006;567:79-86.
14. Minning T, Lytle DA, Pham M, Kelty K. Systematic evaluation of dissolved lead sorption losses to particulate syringe filter materials. Environ Monit Assess 2015;187:383.
15. Ahmad R, Kookana RS, Alston AM. Syringe Filtration as a source of error in pesticide residue analysis in environmental samples. Bulletin of Environmental Contamination and Toxicology 2001;66:313-8.
16. Carlson M, Thompson RD. Analyte loss due to membrane filter adsorption as determined by high-performance liquid chromatography. J Chromatogr Sci 2000;38:77-83.
17. Heimann AC, Jakobsen R. Filtration through nylon membranes negatively affects analysis of arsenic and phosphate by the molybdenum blue method. Talanta 2007;72:839-41.
18. Liu L, Randolph TW, Carpenter JF. Particles shed from syringe filters and their effects on agitation-induced protein aggregation. J Pharm Sci 2012;101:2952-9.
19. Mitev D, Peshev D, Peev G, Peeva L. Depot effect of bioactive components in experimental membrane filtrations. J Phys : Conf Ser 2017;780:012005.
20. Chen H, Gao B, Li H, Ma LQ. Effects of pH and ionic strength on sulfamethoxazole and ciprofloxacin transport in saturated porous media. J Contam Hydrol 2011;126:29-36.
21. Gibson R, Durán-Álvarez JC, Estrada KL, Chávez A, Jiménez Cisneros B. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010;81:1437-45.
22. Rauch-Williams T, Hoppe-Jones C, Drewes JE. The role of organic matter in the removal of emerging trace organic chemicals during managed aquifer recharge. Water Res 2010;44:449-60.
23. Ternes TA, Meisenheimer M, McDowell D, et al. Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 2002;36:3855-63.
24. Xing B, Pignatello JJ. Time-dependent isotherm shape of organic compounds in soil organic matter: Implications for sorption mechanism. Environ Toxicol Chem 1996;15:1282-8.
25. Xing B, Pignatello JJ, Gigliotti B. Competitive sorption between atrazine and other organic compounds in soils and model sorbents. Environ Sci Technol 1996;30:2432-40.
26. Leboeuf EJ, Weber WJ. A distributed reactivity model for sorption by soils and sediments. 8. sorbent organic domains: discovery of a humic acid glass transition and an argument for a polymer-based model. Environ Sci Technol 1997;31:1697-702.
27. Chandramouli B, Benskin JP, Hamilton MC, Cosgrove JR. Sorption of per- and polyfluoroalkyl substances (PFASs) on filter media: implications for phase partitioning studies. Environ Toxicol Chem 2015;34:30-6.
28. Labadie P, Chevreuil M. Biogeochemical dynamics of perfluorinated alkyl acids and sulfonates in the River Seine (Paris, France) under contrasting hydrological conditions. Environ Pollut 2011;159:3634-9.
29. Wu D, Sui Q, Yu X, et al. Identification of indicator PPCPs in landfill leachates and livestock wastewaters using multi-residue analysis of 70 PPCPs: Analytical method development and application in Yangtze River Delta, China. Sci Total Environ 2021;753:141653.
30. Ahrens L, Taniyasu S, Yeung LW, et al. Distribution of polyfluoroalkyl compounds in water, suspended particulate matter and sediment from Tokyo Bay, Japan. Chemosphere 2010;79:266-72.
31. Baduel C, Paxman CJ, Mueller JF. Perfluoroalkyl substances in a firefighting training ground (FTG), distribution and potential future release. J Hazard Mater 2015;296:46-53.
32. Hytteborn JK, Temnerud J, Alexander RB, et al. Patterns and predictability in the intra-annual organic carbon variability across the boreal and hemiboreal landscape. Sci Total Environ 2015;520:260-9.
33. Hillebrand O, Musallam S, Scherer L, Nödler K, Licha T. The challenge of sample-stabilisation in the era of multi-residue analytical methods: a practical guideline for the stabilisation of 46 organic micropollutants in aqueous samples. Sci Total Environ 2013;454-455:289-98.
34. Tran NH, Hu J, Ong SL. Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC-MS/MS and isotope dilution. Talanta 2013;113:82-92.
35. Fox JW. Sample preparation in biological mass spectrometry. J Am Soc Mass Spectrom 2012;23:1440.
36. Godby N, Conklin A. Comparing adsorption of bisphenol A and similar compounds in aqueous solution by syringe filters. Adsorption Science & Technology 2016;35:153-61.
37. Hebig KH, Nödler K, Licha T, Scheytt TJ. Impact of materials used in lab and field experiments on the recovery of organic micropollutants. Sci Total Environ 2014;473-474:125-31.
38. Wang S, Li J, Suo J, Luo T. Surface modification of porous poly(tetrafluoraethylene) film by a simple chemical oxidation treatment. Applied Surface Science 2010;256:2293-8.
39. Wang F, Zhu H, Zhang H, et al. Effect of surface hydrophilic modification on the wettability, surface charge property and separation performance of PTFE membrane. Journal of Water Process Engineering 2015;8:11-8.
40. Nghiem L, Schäfer A, Waite T. Adsorptive interactions between membranes and trace contaminants. Desalination 2002;147:269-74.
41. Cartinella JL, Cath TY, Flynn MT, et al. Removal of natural steroid hormones from wastewater using membrane contactor processes. Environ Sci Technol 2006;40:7381-6.
42. Taheran M, Brar SK, Verma M, et al. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci Total Environ 2016;547:60-77.
43. Braeken L, Ramaekers R, Zhang Y, et al. Influence of hydrophobicity on retention in nanofiltration of aqueous solutions containing organic compounds. Journal of Membrane Science 2005;252:195-203.
44. Han J, Qiu W, Gao W. Adsorption of estrone in microfiltration membrane filters. Chemical Engineering Journal 2010;165:819-26.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].