Contaminants of emerging concern and aquatic organisms: the need to consider hormetic responses in effect evaluations
Abstract
Contaminants of emerging concern are widespread in the world’s waters, raising concerns regarding their effects on living organisms. To evaluate the effects of and predict risks associated with such chemicals, dose-response studies are needed, while the nature of the dose-response relationship is critical for the outcomes of such evaluations. Here, we summarize the literature reporting hormetic responses of aquatic organisms to contaminants of emerging concern. Hormesis is a biphasic dose response encompassing stimulatory responses to low doses and inhibitory responses to high doses. We demonstrate that it occurs widely in numerous aquatic organisms exposed to a wide array of contaminants, including nano/microplastics, suggesting potential effects at doses/concentrations that are considerably lower than the traditional toxicological threshold, which cannot be identified or predicted unless hormesis is considered in the study design. To tackle the effects and associated risks of nano/microplastics and other contaminants on aquatic organisms, hormesis should therefore be taken into account early in the design of studies as well as in relevant risk assessments.
Keywords
The liquid form of water not only is essential for human health[1] but is also fundamental for carbon-based life, i.e., the form of life on Earth that we currently know[2]. Even though two thirds of the surface area of Earth is covered with oceans, only 3% of the Earth’s water is freshwater[3]. Furthermore, 99% of the liquid freshwater represents groundwater, and, thus, only 1% of the freshwater volume is accessible in lakes, reservoirs, and rivers[3]. Seventy percent of freshwater is used for agriculture in most regions of the world[4]. An about 60% increase of food production between the mid-2000s and 2050 will be needed; however, the world’s water demand is predicted to increase by 55%[4]. Hence, preserving water quality is of paramount importance for the sustainability of the biosphere.
While numerous chemicals are essential in modern life, their extensive use leads to contamination of the water cycle, which is expected to increase with age, health, growth, and living standards of humans[5]. Numerous new chemicals are introduced yearly from industry and commerce as well as from consumption in the urban water cycle[4]. A study estimated that only 330 million people, at most, had access to safe water in China by 2010, and three quarter of the rural water sources were not considered safe by national standards[6]. There are numerous potential sources of water pollution, such as urban streets, suburban development, wastewater treatment plants, factories, croplands, animal feedlots, and rural homes, leading to extensive contamination of natural systems too[4]. Water quality and availability are expected to decrease due to climate change, indicating an increased need for reclaimed water from wastewater treatment for multiple purposes, yet effluent treatment is limited in its ability to remove contaminants of emerging concern[5]. Therefore, it is profoundly important to understand the effects of such contaminants on living organisms as well as their ecological risks. The assessment of the effects and risks associated with contaminants requires dose-response studies, the outcomes of which depend on the nature of the dose-response relationship. Hence, this commentary is aimed at summarizing hormetic responses of aquatic organisms to contaminants of emerging concern.
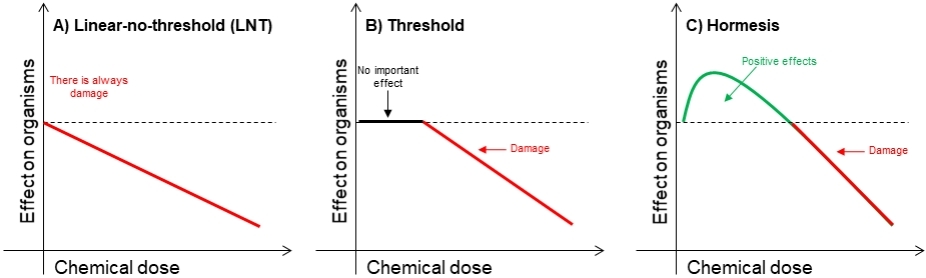
The literature on (eco)toxicological research has been largely framed within a linear-no-threshold (LNT) perspective throughout the 20th century, leading to the wide adoption of the LNT dose-response model by agencies in the United States as well as around the world[7-9]. According to the LNT model, chemical-induced damage increases proportionally to the dose (Figure 1A; hereafter. dose refers to concentration, exposure, or amount unless specified otherwise). LNT assumes that: (1) the damage is cumulative and irreversible; (2) there are no damage-repair mechanisms; (3) organisms lack any capacity to defend and protect themselves against environmental challenges; and (4) each single chemical molecule and the tiniest dose can produce damage up to some extent. The studies upon which the LNT is developed and widely adopted commonly include only few and typically very high doses that are usually environmentally irrelevant.
Figure 1. A hypothetical illustration of different dose responses: linear-no-threshold (A); threshold (B); and hormesis (C).
Along with LNT, the threshold model is one of the two most widely-used dose-response models in the scientific literature and broadly adopted in worldwide regulatory risk assessments for non-carcinogen chemicals of human health concern, and especially for ecological risk assessment[10-12]. The threshold model is characterized by an initially null response that extends up to a certain dose, which is considered as the toxicological threshold, and then followed by damage proportionally to dose similarly to the LNT model [Figure 1B]. Any potential effect of doses smaller than the toxicological threshold is attributed to random variance, due to, e.g., measurement, experimental, and/or environmental errors. Conversely to LNT, the threshold model refuses that each single molecule or the tiniest dose induces damage. It also denies the possibility that important responses of organisms can occur at doses smaller than the toxicological threshold.
In the recent years, however, hormesis has been brought into the mainstream of dose response science[13]. Hormesis is a dose response that is biphasic in nature, eventually having two distinct phases of responses/effects [Figure 1C]. The one phase represents responses that are commonly stimulatory and appears left to the no-observed-adverse-effect-level (NOAEL; toxicological threshold), whereas the other phase depicts negative effects (damage) and appears to the right of the NOAEL. Depending on the endpoint evaluated, it can be U- or J-shaped or their inverse[14]. Hormesis suggests that: (1) the damage is cumulative only after a threshold and is reversible; (2) there are damage-repair mechanisms; (3) organisms have the capacity to defend and protect themselves against environmental challenges; and (4) only doses larger than a threshold dose (NOAEL) can produce damage. It is therefore in striking contrast with the LNT model and to a lesser extent with the threshold model.
Hormesis was meant to climb the Hill at Golgotha from early in its inception as a scientific concept due to the - demonstrably incorrect - link with homeopathy, preventing its advancement throughout the 20th century[13]. Therefore, hormesis has scientifically matured mainly over the last two decades[13]. The current state of the art covers five major advancement areas: (1) documentation of its widespread occurrence and frequency in numerous organisms as a result of exposure to a plethora of chemical agents; (2) understanding of its generalized quantitative features (e.g., typically up to 60% stimulation) and their independency from the underlying mechanisms, stress-inducing agents, and organisms; (3) development of mathematical models for its statistical evaluation; (4) improvement of the understanding of the underlying mechanisms and potential associated ecological risks; and (5) recognition of its support by ecological and evolutionary theory[13-29].
Hormetic responses induced by aquatic contaminants/pollutants[1] in aquatic organisms have been documented since a long time ago. Pollutants are contaminants occurring at levels that can become hazardous to health and are commonly regulated. Readers may also refer to the Safe Drinking Water Act
Today, the evidence of hormesis induced by environmental pollutants and contaminants of emerging concern in aquatic organisms has considerably expanded. For example, a review of the literature concerning the effect of engineered nanomaterials on algae identified 46 hormetic-like dose responses[34]. Another detailed review of the published literature revealed an array of studies reporting hormetic responses of various terrestrial and aquatic creatures to micro- and nanoplastics[35], which are also supported by the findings of recent meta-analyses of the effects of microplastics on aquatic organisms, with significant hormetic responses to environmentally realistic concentrations, not only for genotoxicity but also for reproduction endpoints[36,37].
A further survey of the literature for the purpose of this study revealed ample evidence suggestive of hormesis for numerous aquatic organisms and various pollutants and emerging contaminants in dozens of publications, of which only a few selected examples are cited here (older examples can also be traced in the therein references). These examples include a plethora of species, such as of algae (microphytes)[38-49], aquatic flowering plants[50], organism-attached biofilms[51], crustaceans[52-57], cyanobacteria[49,58-61], fishes[62-69], macrophytes[70-75], marine polychaete[76], mollusks (e.g., clams and mussels)[77,78], periphyton[79], phytoplankton[80], sea anemones[81,82], and snails[83]. Responses suggestive of hormesis were found for molecular (molecules involved in oxidative stress), cellular (e.g., growth and density), and whole-organism (e.g., growth and body mass) endpoints, as well as endpoints suggesting potential sub-NOAEL effects on organismic interactions, such as via altered feeding activity[83]. Such responses were induced by chemicals such as antibiotics/antifungals[38,39,42,45,50,51,67,70,71,75], steroid hormones[41,83], and other human drugs[63,69], bisphenol A and its substitutes[66], chemical leached from disposed light sticks[55], electroplating process-emitted particulate matter[40], effluents from textile-dyeing wastewater treatment plants[46], fullerene crystals (nC60)[57], metals and ionic liquids[62,77,81,82], micro/nanoplastics and their leachates[43,44,47,53,60,64,80,84], engineered nanomaterials[58,59,61,65], pesticides[48,49,52,56,72-74,79,84], phthalic acid esters[78], and polybrominated diphenyl ethers[54]. These chemicals include many major contaminants of emerging concern and were applied either individually or jointly, indicating the potential occurrence of hormesis in aquatic organisms exposed to mixtures of such chemicals. Hormetic responses, however, are known to exhibit a significant temporal variation, including overcompensation stimulation, which suggests that a dose-time component needs to be further studied in relation to the response of aquatic organisms to contaminants of emerging concern in the future.
The literature survey and detailed review of the evaluated publications revealed that, in some key studies with findings of hormetic responses, hormesis was not acknowledged, while in other publications hormesis was claimed without sufficient evidence permitting such a conclusion. This suggests that, while hormesis acknowledgement is increased in the aquatic-related literature, there is still room for better understanding when it is present and when not. To tackle this issue and facilitate our understanding, it is suggested that researchers who are not immersed into the hormesis literature refer to some key review papers on the topic before drawing absolute conclusions on whether their results reflect hormesis.
In the light of the accumulated evidence for widespread occurrence of hormetic responses across aquatic organisms, hormetic response should be considered when evaluating the effects of micro/nanoplastics and other contaminants of emerging concern on aquatic organisms as well as in ecological risk assessments[85]. While the implications of hormetic responses to such contaminants for populations or communities of aquatic organisms are completely unknown, more information about the low-dose, sub-NOAEL effects is needed in order to feed future regulatory risk assessments and enhance the scientific understanding of the underlying mechanisms in aquatic organisms.
The herein analysis also indicates the need for improved research designs that will have the capacity to identify or predict hormetic responses, especially by including a higher number of doses in the sub-NOAEL zone. The maximum stimulation increases by 7% for each dose additionally included in the sub-NOAEL zone[86], indicating that the number of doses included in the sub-NOAEL zone affects the effect estimates. To minimize error from underestimation of sub-NOAEL effects, a minimum of six doses should be included in the sub-NOAEL zone[86]. Care should be exercised to include sub-NAOEL doses differing by orders of magnitude between them. Furthermore, sub-NOAEL responses are modest in amplitude, commonly not greater than 60% different from the control response[86], which makes their statistical detection difficult[19]. Hence, it is important that studies directed to study hormetic responses have robust experimental design with enhanced statistical power, indicating the need for a sufficient number of samples and/or experimental units. Finally, the temporal variation in the hormetic responses indicates the need for multiple assessments over time. The employment of a dependent-samples design with multiple levels could further improve the statistical power.
Although there is a perspective for developing chemicals exhibiting quick and complete degradation in the environment[5], a more rapid degradation in the environment might lead to higher exposures of living organisms due to spontaneously higher levels of bioavailable contaminants. Such spontaneous exposures, whether leading to positive or negative responses of individual organisms, could have unpredicted ecological implications. This exposition, by demonstrating the wide occurrence of responses to sub-NOAEL responses that are also more environmentally realistic, reinforces the need that agendas targeting chemical safety extend to natural ecosystems[87].
DECLARATIONS
Acknowledgement
Agathokleous E acknowledges multi-year support from The Startup Foundation for Introducing Talent of Nanjing University of Information Science & Technology (NUIST), Nanjing, China (No. 003080). Calabrese EJ acknowledges longtime support from the US Air Force (No. AFOSR FA9550-13-1-0047) and ExxonMobil Foundation (No. S18200000000256). The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing policies or endorsement, either expressed or implied. Sponsors were not involved in study design, collection, analysis, interpretation, writing, and decision to and where to submit for publication consideration.
Authors’ contributions
Reviewed literature, drafted the manuscript, had a leading role, served as the hub of communication among the authors, and supervised the production of the manuscript: Agathokleous E
Reviewed the manuscript and contributed intellectual input: Barceló D, Fatta-Kassinos D, Moore MN, Calabrese EJ
Checked and approved the final version for publication: Agathokleous E, Barceló D, Fatta-Kassinos D, Moore MN, Calabrese EJ.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
REFERENCES
1. Jéquier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64:115-23.
4. UN Environment. Global chemicals outlook II from legacies to innovative solutions: implementing the 2030 agenda for sustainable development. United Nations Environment Programme; 2019.
5. Kümmerer K, Dionysiou DD, Olsson O, Fatta-Kassinos D. A path to clean water. Science. 2018;361:222-4.
6. Yang H, Wright JA, Gundry SW. Water accessibility: boost water safety in rural China. Nature. 2012;484:318.
7. Calabrese EJ. Ethical failings: the problematic history of cancer risk assessment. Environ Res. 2021;193:110582.
8. Calabrese EJ. LNT and cancer risk assessment: Its flawed foundations part 2: how unsound LNT science became accepted. Environ Res. 2021;197:111041.
9. Calabrese EJ. The linear No-Threshold (LNT) dose response model: a comprehensive assessment of its historical and scientific foundations. Chem Biol Interact. 2019;301:6-25.
10. Calabrese EJ, Priest ND, Kozumbo WJ. Thresholds for carcinogens. Chem Biol Interact. 2021;341:109464.
11. Tsatsakis A. Toxicological risk assessment and multi-system health impacts from exposure. Elsevier; 2021.
12. Tsatsakis AM, Vassilopoulou L, Kovatsi L, et al. The dose response principle from philosophy to modern toxicology: the impact of ancient philosophy and medicine in modern toxicology science. Toxicol Rep. 2018;5:1107-13.
13. Agathokleous E, Calabrese EJ. Hormesis: the dose response for the 21st century: the future has arrived. Toxicology. 2019;425:152249.
14. Leak RK, Calabrese EJ, Kozumbo WJ, et al. Enhancing and extending biological performance and resilience. Dose Response. 2018;16:1559325818784501.
15. Erofeeva EA. Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci Total Environ. 2022;804:150059.
17. Carvalho MEA, Castro PRC, Azevedo RA. Hormesis in plants under Cd exposure: from toxic to beneficial element? J Hazard Mater. 2020;384:121434.
18. Shahid M, Niazi NK, Rinklebe J, Bundschuh J, Dumat C, Pinelli E. Trace elements-induced phytohormesis: a critical review and mechanistic interpretation. Crit Rev Environ Sci Technol. 2020;50:1984-2015.
19. Agathokleous E, Calabrese EJ. A global environmental health perspective and optimisation of stress. Sci Total Environ. 2020;704:135263.
20. Agathokleous E, Calabrese EJ. Hormesis can enhance agricultural sustainability in a changing world. Glob Food Secur. 2019;20:150-5.
21. Costantini D, Metcalfe NB, Monaghan P. Ecological processes in a hormetic framework. Ecol Lett. 2010;13:1435-47.
22. Costantini D, Monaghan P, Metcalfe NB. Prior hormetic priming is costly under environmental mismatch. Biol Lett. 2014;10:20131010.
23. Costantini D, Borremans B. The linear no-threshold model is less realistic than threshold or hormesis-based models: an evolutionary perspective. Chem Biol Interact. 2019;301:26-33.
25. Calabrese EJ, Kozumbo WJ. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol Res. 2021;167:105526.
26. Kozumbo WJ, Calabrese EJ. Two decades (1998-2018) of research Progress on Hormesis: advancing biological understanding and enabling novel applications. J Cell Commun Signal. 2019;13:273-5.
27. Calabrese EJ. Hormesis commonly observed in the assessment of aneuploidy in yeast. Environ Pollut. 2017;225:713-28.
28. Cedergreen N, Streibig JC, Kudsk P, Mathiassen SK, Duke SO. The occurrence of hormesis in plants and algae. Dose Response. 2006;5:150-62.
29. Moore MN, Shaw JP, Pascoe C, Beesley A, Viarengo A, Lowe DM. Anti-oxidative hormetic effects of cellular autophagy induced by nutrient deprivation in a molluscan animal model. Mar Environ Res. 2020;156:104903.
30. Laughlin RB Jr, Ng J, Guard HE. Hormesis: a response to low environmental concentrations of petroleum hydrocarbons. Science. 1981;211:705-7.
31. Stebbing ARD. The effects of low metal levels on a clonal hydroid. J Mar Biol Ass. 1976;56:977-94.
32. Stebbing A. Hormesis—stimulation of colony growth in Campanularia flexuosa (hydrozoa) by copper, cadmium and other toxicants. Aqua Toxicol. 1981;1:227-38.
33. Stebbing A. Hormesis — the stimulation of growth by low levels of inhibitors. Sci Total Environ. 1982;22:213-34.
34. Agathokleous E, Feng Z, Iavicoli I, Calabrese EJ. The two faces of nanomaterials: a quantification of hormesis in algae and plants. Environ Int. 2019;131:105044.
35. Agathokleous E, Iavicoli I, Barceló D, Calabrese EJ. Micro/nanoplastics effects on organisms: a review focusing on 'dose'. J Hazard Mater. 2021;417:126084.
36. Sun T, Zhan J, Li F, Ji C, Wu H. Evidence-based meta-analysis of the genotoxicity induced by microplastics in aquatic organisms at environmentally relevant concentrations. Sci Total Environ. 2021;783:147076.
37. Sun T, Zhan J, Li F, Ji C, Wu H. Effect of microplastics on aquatic biota: a hormetic perspective. Environ Pollut. 2021;285:117206.
38. Li J, Li W, Min Z, Zheng Q, Han J, Li P. Physiological, biochemical and transcription effects of roxithromycin before and after phototransformation in Chlorella pyrenoidosa. Aquat Toxicol. 2021;238:105911.
39. Mao Y, Yu Y, Ma Z, et al. Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress. Ecotoxicol Environ Saf. 2021;222:112496.
40. Pikula K, Kirichenko K, Vakhniuk I, et al. Aquatic toxicity of particulate matter emitted by five electroplating processes in two marine microalgae species. Toxicol Rep. 2021;8:880-7.
41. Cantalupi A, Maraschi F, Pretali L, et al. Glucocorticoids in freshwaters: degradation by solar light and environmental toxicity of the photoproducts. Int J Environ Res Public Health. 2020;17:8717.
42. Zhang M, Steinman AD, Xue Q, Zhao Y, Xu Y, Xie L. Effects of erythromycin and sulfamethoxazole on Microcystis aeruginosa: Cytotoxic endpoints, production and release of microcystin-LR. J Hazard Mater. 2020;399:123021.
43. Qu H, Ma R, Barrett H, et al. How microplastics affect chiral illicit drug methamphetamine in aquatic food chain? From green alga (Chlorella pyrenoidosa) to freshwater snail (Cipangopaludian cathayensis). Environ Int. 2020;136:105480.
44. Song C, Liu Z, Wang C, Li S, Kitamura Y. Different interaction performance between microplastics and microalgae: the bio-elimination potential of Chlorella sp. L38 and Phaeodactylum tricornutum MASCC-0025. Sci Total Environ. 2020;723:138146.
45. Guo J, Ma Z, Peng J, et al. Transcriptomic analysis of Raphidocelis subcapitata exposed to erythromycin: the role of DNA replication in hormesis and growth inhibition. J Hazard Mater. 2021;402:123512.
46. Cai H, Liang J, Ning XA, Lai X, Li Y. Algal toxicity induced by effluents from textile-dyeing wastewater treatment plants. J Environ Sci (China). 2020;91:199-208.
47. Chae Y, Kim D, An YJ. Effects of micro-sized polyethylene spheres on the marine microalga Dunaliella salina: focusing on the algal cell to plastic particle size ratio. Aquat Toxicol. 2019;216:105296.
48. Chamsi O, Pinelli E, Faucon B, et al. Effects of herbicide mixtures on freshwater microalgae with the potential effect of a safener. Ann Limnol - Int J Lim. 2019;55:3.
49. Zhang Y, Calabrese EJ, Zhang J, Gao D, Qin M, Lin Z. A trigger mechanism of herbicides to phytoplankton blooms: from the standpoint of hormesis involving cytochrome b559, reactive oxygen species and nitric oxide. Water Res. 2020;173:115584.
50. Guo X, Liu M, Zhong H, et al. Potential of Myriophyllum aquaticum for phytoremediation of water contaminated with tetracycline antibiotics and copper. J Environ Manage. 2020;270:110867.
51. Guo X, Zhu L, Zhong H, Li P, Zhang C, Wei D. Response of antibiotic and heavy metal resistance genes to tetracyclines and copper in substrate-free hydroponic microcosms with Myriophyllum aquaticum. J Hazard Mater. 2021;413:125444.
52. González-Doncel M, Fernández Torija C, Pablos MV, García Hortigüela P, López Arévalo M, Beltrán EM. The role of PFOS on triclosan toxicity to two model freshwater organisms. Environ Pollut. 2020;263:114604.
53. Li Y, Liu Z, Li M, et al. Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J Hazard Mater. 2020;398:122990.
54. Liu Y, Guo R, Tang S, et al. Single and mixture toxicities of BDE-47, 6-OH-BDE-47 and 6-MeO-BDE-47 on the feeding activity of Daphnia magna: From behavior assessment to neurotoxicity. Chemosphere. 2018;195:542-50.
55. Cesar-Ribeiro C. Chemical contents of disposed light sticks affect the physiology of rocky crab Pachygrapsus transversus and gray shrimps Litopennaeus vanammei. Bull Environ Contam Toxicol. 2021;107:370-7.
56. Bordin ER, Cesar Munhoz R, Panicio PP, Freitas AM. Transgenerational effects of environmentally relevant concentrations of atrazine and glyphosate herbicides, isolated and in mixture, to freshwater microcrustacean Daphnia magna. Res Sq. 2021; doi: 10.21203/rs.3.rs-790734/v1.
57. Wang P, Ng QX, Zhang B, et al. Employing multi-omics to elucidate the hormetic response against oxidative stress exerted by nC60 on Daphnia pulex. Environ Pollut. 2019;251:22-9.
58. Xu K, Li Z, Juneau P, et al. Toxic and protective mechanisms of cyanobacterium Synechocystis sp. in response to titanium dioxide nanoparticles. Environ Pollut. 2021;274:116508.
59. Wu S, Ji X, Li X, et al. Mutual impacts and interactions of antibiotic resistance genes, microcystin synthetase genes, graphene oxide, and Microcystis aeruginosa in synthetic wastewater. Environ Sci Pollut Res Int. 2021; doi: 10.1007/s11356-021-15627-2.
60. Wan Q, Li J, Chen Y. Comparative growth and cellular responses of toxigenic Microcystis exposed to different types of microplastics at various doses. Environ Pollut. 2021;290:117950.
61. Zuo S, Yang H, Jiang X, Ma Y. Magnetic Fe3O4 nanoparticles enhance cyanobactericidal effect of allelopathic p-hydroxybenzoic acid on Microcystis aeruginosa by enhancing hydroxyl radical production. Sci Total Environ. 2021;770:145201.
62. Biswas S, Bellare J. Adaptive mechanisms induced by sparingly soluble mercury sulfide (HgS) in zebrafish: behavioural and proteomics analysis. Chemosphere. 2021;270:129438.
63. Constantine LA, Green JW, Schneider SZ. Ibuprofen: fish short-term reproduction assay with Zebrafish (Danio rerio) based on an extended OECD 229 protocol. Environ Toxicol Chem. 2020;39:1534-45.
64. Ding J, Zhang S, Razanajatovo RM, Zou H, Zhu W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ Pollut. 2018;238:1-9.
65. Ding Y, Yang Y, Chen J, Chen H, Wu Y, Jin L. Toxic effects of ZnSe/ZnS quantum dots on the reproduction and genotoxiticy of rare minnow (Gobiocypris rarus). Comp Biochem Physiol C Toxicol Pharmacol. 2021;247:109065.
66. Fan X, Hou T, Jia J, Tang K, Wei X, Wang Z. Discrepant dose responses of bisphenol A on oxidative stress and DNA methylation in grass carp ovary cells. Chemosphere. 2020;248:126110.
67. Han Y, Ma Y, Yao S, Zhang J, Hu C. In vivo and in silico evaluations of survival and cardiac developmental toxicity of quinolone antibiotics in zebrafish embryos (Danio rerio). Environ Pollut. 2021;277:116779.
68. Jin M, Dang J, Paudel YN, et al. The possible hormetic effects of fluorene-9-bisphenol on regulating hypothalamic-pituitary-thyroid axis in zebrafish. Sci Total Environ. 2021;776:145963.
69. Pandelides Z, Thornton C, Lovitt KG, et al. Developmental exposure to Δ9-tetrahydrocannabinol (THC) causes biphasic effects on longevity, inflammation, and reproduction in aged zebrafish (Danio rerio). Geroscience. 2020;42:923-36.
70. Alkimin GD, Santos J, Soares AMVM, Nunes B. Ecotoxicological effects of the azole antifungal agent clotrimazole on the macrophyte species Lemna minor and Lemna gibba. Comp Biochem Physiol C Toxicol Pharmacol. 2020;237:108835.
71. Liu Y, Pang Y, Yang L, Ning S, Wang D, Wu Z. Responses of Hydrocharis dubia (Bl.) Backer and Trapa bispinosa roxb. to tetracycline exposure. Ecotoxicol Environ Saf. 2020;202:110890.
72. Peres L, Della Vechia J, Cruz C. Hormesis effect of herbicides subdoses on submerged macrophytes in microassay conditions. Planta daninha. 2017:35.
73. Di Baccio D, Pietrini F, Bertolotto P, et al. Response of Lemna gibba L. to high and environmentally relevant concentrations of ibuprofen: Removal, metabolism and morpho-physiological traits for biomonitoring of emerging contaminants. Sci Total Environ. 2017;584-585:363-73.
74. Farooq N, Abbas T, Tanveer A, et al. Differential hormetic response of fenoxaprop-p-Ethyl resistant and susceptible phalaris minor populations: a potential factor in resistance evolution. Planta daninha. 2019;37:e019187554.
75. Hu H, Zhou Q, Li X, et al. Phytoremediation of anaerobically digested swine wastewater contaminated by oxytetracycline via Lemna aequinoctialis: nutrient removal, growth characteristics and degradation pathways. Bioresour Technol. 2019;291:121853.
76. Liu F, Lu Z, Wu H, Ji C. Dose-dependent effects induced by cadmium in polychaete Perinereis aibuhitensis. Ecotoxicol Environ Saf. 2019;169:714-21.
77. Zhan J, Wang S, Li F, Ji C, Wu H. Dose-dependent responses of metabolism and tissue injuries in clam Ruditapes philippinarum after subchronic exposure to cadmium. Sci Total Environ. 2021;779:146479.
78. Xu H, Cao W, Sun H, et al. Dose-dependent effects of Di-(2-Ethylhexyl) phthalate (DEHP) in mussel Mytilus galloprovincialis. Front Mar Sci. 2021;8:658361.
79. Vera MS, Trinelli MA. First evaluation of the periphyton recovery after glyphosate exposure. Environ Pollut. 2021;290:117998.
80. Mao Y, Ai H, Chen Y, et al. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere. 2018;208:59-68.
81. Ianna ML, Reichelt-Brushett A, Howe PL, Brushett D. Application of a behavioural and biochemical endpoint in ecotoxicity testing with Exaiptasia pallida. Chemosphere. 2020;257:127240.
82. Howe PL, Reichelt-Brushett AJ, Clark MW. Development of a chronic, early life-stage sub-lethal toxicity test and recovery assessment for the tropical zooxanthellate sea anemone Aiptasia pulchella. Ecotoxicol Environ Saf. 2014;100:138-47.
83. Svigruha R, Fodor I, Padisak J, Pirger Z. Progestogen-induced alterations and their ecological relevance in different embryonic and adult behaviours of an invertebrate model species, the great pond snail (Lymnaea stagnalis). Environ Sci Pollut Res Int. 2021;28:59391-402.
84. Nong QY, Liu YA, Qin LT, et al. Toxic mechanism of three azole fungicides and their mixture to green alga Chlorella pyrenoidosa. Chemosphere. 2021;262:127793.
85. Agathokleous E, Barceló D, Calabrese EJ. US EPA: is there room to open a new window for evaluating potential sub-threshold effects and ecological risks? Environ Pollut. 2021;284:117372.
86. Calabrese EJ, Agathokleous E, Kozumbo WJ, Stanek EJ 3rd, Leonard D. Estimating the range of the maximum hormetic stimulatory response. Environ Res. 2019;170:337-43.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].