Achieving photocatalytic water reduction and oxidation over narrow bandgap FeVO4
Abstract
The exploration of novel oxide photocatalysts with narrow bandgaps is highly desirable for efficient photocatalytic water splitting. However, this is rather challenging as reducing the bandgap generally leads to severe charge recombination in photocatalysts. To address these issues, the present work demonstrates, for the first time, the synthesis and application of triclinic FeVO4 with an absorption edge of 575 nm for visible-light-driven photocatalytic water reduction and oxidation. Based on it, the Cr doping strategy is implemented on the FeVO4 photocatalyst to further promote the charge separation and the photocatalytic water splitting performance, achieving an apparent quantum efficiency (AQE) of 0.26% at 420 nm (± 15 nm) for an O2 evolution reaction. Detailed analysis shows that an impurity level below the conduction band minimum originating from the Cr 3d orbital is formed after Cr doping, facilitating the prolonged absorption edge and the enhanced charge separation. This work inaugurates the application field of the narrow bandgap particulate FeVO4 photocatalyst in photocatalytic water splitting, and validates that charge separation can be promoted by Cr doping, both of which are promising to be further developed for efficient solar energy conversion.
Keywords
INTRODUCTION
As an abundant and sustainable energy source, solar energy shows great potential in the application field of photocatalytic water splitting for green hydrogen production[1-3]. At present, most photocatalysts for photocatalytic water splitting are ultraviolet-light-responsive oxides, such as TiO2, SrTiO3, etc.[4,5]. However, in a photocatalytic process, the light absorption wavelength range of photocatalysts determines the maximum theoretical solar energy conversion efficiency[6,7]. Therefore, the development of narrow bandgap photocatalysts is the prerequisite to achieving an efficient solar water splitting process. To date, a series of visible-light-responsive oxides, nitrides, oxynitrides, sulfides, oxysulfides and selenides, including g-C3N4, BiVO4, Ta3N5, BaTaO2N, Y2Ti2O5S2, (ZnSe)x(CuGa2.5Se4.25)1-x, etc., have been demonstrated for photocatalytic water splitting[8-14]. Among those reported semiconductors, the oxides show the best thermal stability in the air and excellent photostability under illumination. However, currently, the number of demonstrated oxide photocatalysts with narrow bandgaps remains limited, especially for those capable of simultaneously achieving photocatalytic hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Another issue with narrow bandgap photocatalysts is that as the bandgap of the semiconductor decreases, severe charge recombination generally occurs, resulting in poor charge separation and low efficiency of photocatalytic HER and OER. Therefore, developing oxide photocatalysts with narrow bandgaps while possessing sufficient charge separation is still challenging for efficient solar-driven photocatalytic water splitting[15-18].
The iron vanadate (FeVO4) is a narrow bandgap semiconductor with an absorption edge of 575 nm. Band structure analysis shows that its levels of the conduction band minimum (CBM) and the valance band maximum (VBM) are at -0.37 and 1.73 V [vs. normal hydrogen electrode (NHE)], respectively, which straddle the potentials of H+/H2 (0 V vs. NHE) and O2/H2O (1.23 V vs. NHE)[19-23]. It means that FeVO4 meets the requirement for both photocatalytic HER and OER in thermodynamics. Up until now, various applications, such as photoelectrochemical (PEC) water splitting[24,25] and photocatalytic degradation of organic pollutants[26,27], have been investigated over FeVO4 photo(electro)catalysts, demonstrating its excellent properties in optoelectronics. However, there is currently no report of particulate FeVO4 photocatalysts for water splitting reaction. In addition, regarding the promotion of charge separation, series strategies, including doping of metal or nonmetal[28,29] and heterojunction construction[30,31], have been demonstrated to be effective for FeVO4 photo(electro)catalysts. For example, it has been reported that the charge separation efficiency can be significantly enhanced by Cr doping over FeVO4 photoanodes for PEC water splitting[32]. Therefore, it is anticipated that the promoted charge separation can also be achieved over FeVO4 photocatalyst by using Cr as a dopant to further improve the water splitting activity.
In this work, we demonstrated that visible-light-driven water reduction and oxidation reactions could be realized over the triclinic FeVO4 with an absorption edge of 575 nm loaded with proper cocatalysts. As far as we know, this is the first report of photocatalytic water splitting for the FeVO4 photocatalysts. Based on this result, it was further identified that the promoted charge separation and enhanced photocatalytic water splitting performance could be achieved by implementing the Cr doping strategy to the FeVO4 photocatalysts, in which an apparent quantum efficiency (AQE) of 0.26% at 420 nm (± 15 nm) was obtained for an OER. Detailed characterization results manifested that the Cr doping could produce an additional impurity level below the CBM of the FeVO4 semiconductor, which could facilitate the prolonged absorption edge and inhibit charge recombination. Our results demonstrate the feasibility of FeVO4 in achieving the photocatalytic water reduction and oxidation reactions, based on which the effectiveness of Cr doping in enhancing the charge separation is also illustrated.
EXPERIMENTAL
Synthesis of FeVO4
The FeVO4·1.1H2O precursor was synthesized via a hydrothermal method, followed by calcination in air to obtain FeVO4[19]. Typically, 10 mL of NH4VO3 (99%, Rhawn Chemical Reagent) aqueous solution (0.3 M) was added to 10 mL of Fe(NO3)3·9H2O (99.5%, Macklin Chemical Reagent) aqueous solution (0.3 M). After continuously stirring for 0.5 h, the resulting suspension was transferred to a 100 mL Teflon-lined stainless-steel autoclave and heated at 453 K for 3 h. The FeVO4·1.1H2O precursor was collected by centrifugation, washed with deionized water several times and then dried in vacuum at 333 K for 6 h. Subsequently, the precursor was calcined in a muffle furnace at 823 K for 2 h to obtain FeVO4.
Synthesis of FeVO4:Cr
FeVO4:Cr [the molar ratio of Cr/(Cr + Fe) is 0, 1.5%, 2.0%, 2.5%, 3.0% or 3.5%] was synthesized in a similar way to FeVO4[33]. The Cr source [Cr(NO3)3·9H2O, 99%, Macklin Chemical Reagent] was added to the solution containing NH4VO3 and Fe(NO3)3·9H2O for Cr doping. Then, the same hydrothermal treatment was implemented to obtain FeVO4:Cr·1.1H2O. After that, FeVO4:Cr·1.1H2O was similarly calcined to obtain FeVO4:Cr.
Preparation of CoOx/FeVO4 and CoOx/FeVO4:Cr by an impregnation method
Typically, 0.1 g of FeVO4 or FeVO4:Cr sample was dispersed in 1.0 mL of aqueous solution containing a calculated amount of [Co(NO3)2·6H2O (99.9%, Aladdin Chemical Reagent)] and evaporated with a water bath. The collected powder was calcined at 573 K for 1 h.
Preparation of CoOx/FeVO4 by a photodeposition method
Typically, 0.1 g of FeVO4 sample was dispersed in 100 mL of aqueous solution containing calculated amounts of Co(NO3)2·6H2O and 20 mM of NaIO3 (99.9%, Aladdin Chemical Reagent). The suspension was evacuated to completely remove the dissolved air and then exposed to visible light (λ ≥ 420 nm) with continuous stirring for 2 h. The temperature of the reaction solution was maintained at 283 K by a chiller. After the reaction, the as-loaded powder was collected by filtration and dried naturally.
Preparation of Pt/FeVO4 and Pt/FeVO4:Cr by an impregnation-reduction method
Typically, 0.1 g of FeVO4 or FeVO4:Cr sample was dispersed in 1.0 mL of aqueous solution containing a calculated amount of H2PtCl6 (99.5%, Sinopharm Chemical) and totally evaporated with a water bath. The collected powder was calcined at 573 K for 2 h under Ar flow (100 mL/min). The resulting powder was added to 30 mL of NaBH4 (98%, Energy Chemical Reagent) aqueous solution (0.2 mg/mL) to reduce the Pt4+ to Pt0. After stirring for 10 min, the solution was filtered and dried in vacuum at 333 K for 6 h.
Photocatalytic H2 evolution reaction
The photocatalytic HER was conducted in a Pyrex top-irradiation-type reactor vessel connected to a gas-closed-circulation system. Typically, 0.1 g of Pt/FeVO4 or Pt/FeVO4:Cr was added to 100 mL of aqueous solution containing ascorbic acid (10 mM, C6H8O6, 99%, Aladdin Chemical Reagent) as a hole sacrificial reagent. The reactor was then connected to the system and thoroughly evacuated. The temperature of the reaction solution was maintained at about 283 K by a chiller. A 300 W Xenon lamp with a cut-off filter (λ ≥ 420 nm) was used as a light source, and the produced H2 gas was analyzed by online gas chromatography (Shimadzu, GC-2014, Ar as carrier gas).
Photocatalytic O2 evolution reaction
The photocatalytic OER was carried out in the same gas-closed-circulation system with HER. Typically,
Measurement of AQE
An AQE was measured using a 300 W Xe lamp with a band-pass filter (Beijing Perfectlight, UVCUT420) to illuminate a Pyrex top-irradiation-type reaction vessel, which was calculated by
where A is a coefficient, which is 4 for oxygen evolution. R is the rate of oxygen evolution, and I is the number of photons measured at 420 nm. The total number of the incident photons was measured to be
Preparation of FeVO4 and FeVO4:Cr photoelectrodes
The photoelectrodes were prepared using an electrophoresis method. Firstly, 25 mg of FeVO4 or FeVO4:Cr (2.5%) was dispersed to 25 mL of acetone solution containing 10 mg of iodine. Two parallel pieces of fluorine-doped tin oxide (FTO) glass were immersed in the suspension. The positive and negative electrodes of the potentiostat were connected with the two FTO glass pieces, respectively, and a constant voltage of 30 V was applied for 5 min. The catalyst powder was uniformly deposited on the negative electrode. After the electrophoretic deposition, the photoelectrode was calcined at 573 K for 1 h to remove the charged species adsorbed on the surface of photocatalysts.
PEC measurements
The PEC measurements were conducted in a three-electrode system on an electrochemical workstation (CHI760E) by using a FeVO4 or FeVO4:Cr photoelectrode as the working electrode, a platinum sheet as the counter electrode, and an Ag/AgCl electrode (saturated KCl) as the reference electrode. Mott-Schottky test was performed in Na2SO4 solution (0.5 M) at a frequency of 500 or 1,000 Hz and a voltage from -1.0 to 1.0 V (vs. Ag/AgCl). Photocurrent density test was performed in Na2SO3 solution (0.5 M) and at a voltage of 0.5 V (vs. Ag/AgCl) by using a 300 W Xenon lamp with a cut-off filter (λ ≥ 420 nm) as a light source. The measured potential at the Ag/AgCl electrode was converted to NHE using the Nernst equation.
Computational method
First-principles calculations were performed by the Vienna ab initio simulation package (VASP)[34,35]. The generalized gradient approximation (GGA) of Perdew-Burke-Ernzerhof (PBE) was employed to describe the exchange-correlation functional[36]. The cut-off energy for the plane wave basis was set to 400 eV and the Monkhorst-pack k-point mesh was set to 4 × 4 × 4. The structural relaxation was implemented until the forces were less than 0.01 eV/Å and the energy convergence of 10-5 eV was obtained.
To simulate the FeVO4:Cr, an Fe atom in the FeVO4 unit cell was replaced by the Cr atom. The Hubbard-type U correction for the strong-correlation d electrons of Fe, V and Cr was set to 3.5, 4.3 and 3.8 eV, respectively[37].
Characterization
The X-ray diffraction (XRD, Rigaku, Smart Lab) with Cu Kα radiation (λ = 1.5418 Å, 40 mA, 40 kV) was employed to investigate the crystalline structure of the prepared samples. The ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS, Shimadzu, UV–2600i) was applied to gain insight into the optical property. A scanning electron microscope (SEM, JEOL, JSM-7800F) and a transmission electron microscope (TEM, JEOL, JEM-2800) were used to determine the morphology and elemental distribution of the catalysts. X-ray photoelectron spectroscopy (XPS, Thermo Scientific, ESCALAB 250Xi, the C 1s peak at 284.8 eV served as the internal standard for calibrating the binding energy) was performed to analyze the valence state of the catalyst surface. The chemical structure of the materials was characterized using microscopic confocal Raman spectroscopy (Raman, TEO, SR-500I-A) with a laser wavelength of 532 nm. The photoluminescence (PL) spectroscopy (Hitachi, F-7000) was used to determine the charge separation efficiency with an excitation wavelength of 310 nm. The Cr content was analyzed using an inductively coupled plasma optical emission spectrometer (ICP-OES, Thermo Fisher iCAP PRO).
RESULTS AND DISCUSSION
Characterization of FeVO4 and photocatalytic performance
Typically, the FeVO4·1.1H2O precursor was prepared by the hydrothermal method, followed by the calcination treatment in the air to obtain FeVO4 [Scheme 1][19]. As given in Supplementary Figure 1, the XRD pattern shows that the diffraction peaks of the FeVO4 sample correspond to the standard pattern of the triclinic FeVO4 (JCPDS#71-1592). A SEM image shows that the FeVO4·1.1H2O precursor possesses a smooth needle-like morphology with a length of about 3-4 μm and a diameter of about 100 nm
Figure 1. (A) UV-Vis DRS and (B) a schematic illustration of the band structure of FeVO4; (C) Time courses of the OER over FeVO4 photocatalysts loaded with CoOx cocatalyst in different methods; (D) Time course of the HER over FeVO4 photocatalyst loaded with Pt cocatalyst. Reaction conditions of (C): 0.1 g of photocatalyst (0.8 wt% Co is loaded); 100 mL of AgNO3 aqueous solution (50 mM); 0.1 g of La2O3; 300 W Xe lamp with a cut-off filter (λ ≥ 420 nm). Reaction conditions of (D): 0.1 g of photocatalyst (1.0 wt% Pt is loaded);
Thus, the application of photocatalytic water splitting over FeVO4 is investigated. For the OER, an oxidation cocatalyst of CoOx was deposited on the FeVO4 by the impregnation (Imp) method and photodeposition (PD) method before the evaluation (see the Experimental Section for details). As provided in Figure 1C, visible-light-driven photocatalytic OER can be achieved over the two photocatalysts, in which CoOx/FeVO4 prepared by the Imp method shows higher activity. For the HER, stable photocatalytic performance under visible light irradiation can also be realized over the FeVO4 photocatalyst loaded with the reduction cocatalyst of Pt [Figure 1D]. Therefore, based on these results, it is concluded that both photocatalytic water oxidation and reduction reactions can be achieved over the FeVO4 photocatalysts under visible light irradiation. To the best of our knowledge, this is the first report on the application of FeVO4 for photocatalytic water splitting.
Doping strategy to promote the FeVO4 photocatalysts
Although the as-developed FeVO4 photocatalyst in this work can absorb visible light with wavelengths as long as 575 nm, the photocatalytic water splitting activity remains low, probably due to poor charge separation. To address this issue, element doping is investigated on the FeVO4 photocatalyst water splitting[33]. Herein, the Cr-doped FeVO4·1.1H2O (denoted as FeVO4:Cr·1.1H2O) was prepared by adding a Cr source in the hydrothermal process, and then the calcination treatment in the air was implemented to obtain the FeVO4:Cr sample (see the Experimental Section for details). In the following text, the molar ratio of Cr/(Cr + Fe) in FeVO4:Cr is 2.5%, unless otherwise specified.
Subsequently, the structure and distribution of elements in FeVO4:Cr·1.1H2O and FeVO4:Cr samples were further investigated to verify the success of the doping. Inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis indicates that Cr has been successfully introduced into FeVO4
where ν is the Raman vibration frequency and R is the estimated bond length[40,41]. In other words, the Raman vibration frequency is inversely proportional to the bond length. The peaks at 720 and 822 cm-1 ascribing to bridging V-O···Fe bond stretching mode shift to higher wavenumbers after Cr doping, indicating that the length becomes shorter [Supplementary Figure 9]. This is consistent with the results of the data for the lattice parameters. UV-Vis DRS shows that the Cr doping can induce a slight redshift of the absorption edge from 575 to 585 nm for the FeVO4-based samples [Supplementary Figure 10]. These results indicate that Cr was successfully doped into FeVO4, widening the absorption edge.
Apart from the effect of Cr doping on the structure and morphology, its influence on the chemical state of the elements after Cr doping was also examined. As shown in Supplementary Figure 11A, the high-resolution Cr 2p XPS of FeVO4 and FeVO4:Cr samples reveal two characteristic peaks of FeVO4:Cr at 576.5 and 587.2 eV corresponding to Cr3+ 2p3/2 and Cr3+ 2p1/2, respectively, indicating that the valence state of Cr is consistent with Fe in FeVO4[42]. In the meantime, two peaks at the same binding energies of 711.2 and
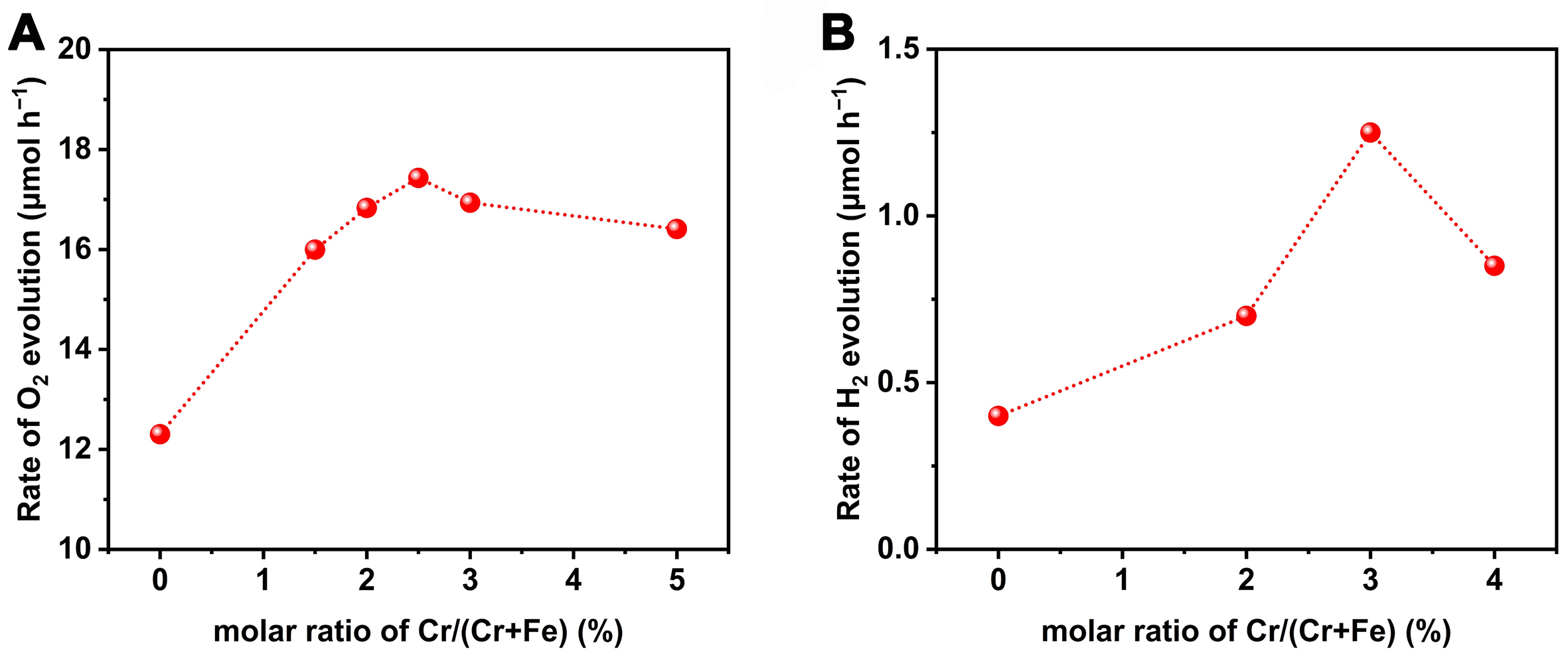
As Cr was successfully doped into FeVO4, the photocatalytic activity of FeVO4:Cr was further investigated. For the OER, under the optimized Cr doping proportion [Figure 2A] and the loading condition of CoOx cocatalyst [Supplementary Figure 12], the maximum O2 evolution rate of CoOx/FeVO4:Cr [the molar ratio of Cr/(Cr + Fe) is 2.5%] is 17.6 μmol/h, higher than that of CoOx/FeVO4 (12.1 μmol/h). The corresponding AQE for OER over the CoOx/FeVO4:Cr photocatalyst is 0.26% at 420 nm (± 15 nm). In addition, the Cr doping strategy for the promoted photocatalytic activity has also been similarly confirmed in another case of HER. As can be seen from Figure 2B, as the molar ratio of Cr/(Cr + Fe) increases from 0% to 4%, the H2 evolution rate follows a volcano-shaped tendency with a maximum value at the ratio of 3%, about three times as high as that of the undoped photocatalyst. Herein, the optimized loading proportion of Pt is
Figure 2. Effect of different molar ratios of Cr/(Cr + Fe) on (A) the photocatalytic O2 evolution rate or (B) the photocatalytic H2 evolution rate over FeVO4. Reaction conditions for (A): 0.1 g of photocatalyst (0.8 wt% Co is loaded); 100 mL of AgNO3 aqueous solution (50 mM); 0.1 g of La2O3; 300 W Xe lamp with a cut-off filter (λ ≥ 420 nm). Reaction condition for (B): 0.1 g of photocatalyst (1.0 wt% Pt is loaded); 100 mL of ascorbic acid aqueous solution (10 mM); 300 W Xe lamp with a cut-off filter (λ ≥ 420 nm).
The origin of Cr doping for the enhanced photocatalytic performance
To determine the influence of Cr dopants on the photogenerated charge separation, firstly, PEC characterization is applied to compare their photocurrent densities in Na2SO3 solution under visible light irradiation [Figure 3A]. In this circumstance, the effect of charge injection efficiency can be excluded due to the existence of the hole scavenger. Considering both samples of FeVO4 and FeVO4:Cr have similar absorption edges, the difference in the photocurrent density is primarily attributed to the distinct charge separation. It can be obviously observed that FeVO4:Cr photoanode exhibits higher photocurrent density than FeVO4 photoanode, indicating the former possesses higher charge separation efficiency. Such a conclusion can be further identified by the PL spectra. As shown in Figure 3B, an emission peak at approximately 625 nm is detected for both samples, in which the FeVO4:Cr sample exhibits a much lower peak intensity, suggesting that charge recombination can be effectively inhibited by the Cr doping treatment.
Figure 3. (A) The photocurrent densities of FeVO4 and FeVO4:Cr photoanodes at 1.0 V vs. NHE in Na2SO3 aqueous solution under visible light ( ≥ 420 nm); (B) PL spectra of FeVO4 and FeVO4:Cr samples. NHE: Normal hydrogen electrode; PL: photoluminescence.
As is known, photocatalytic efficiency is determined by three basic processes: light absorption, charge separation, and surface catalytic conversion. Herein, two factors, other than charge separation, are further investigated to determine their contribution to the enhancement of photocatalytic activity. The absorption edge can be slightly extended by the Cr doping over the FeVO4 semiconductor, which can enhance the light-harvesting efficiency to a certain degree. Regarding surface catalytic conversion, the loaded CoOx cocatalyst is mainly studied. It is confirmed the binding energies at 781.4 eV (2p3/2) and 797.3 eV (2p1/2) in the Co 2p XPS data of both CoOx/FeVO4 and CoOx/FeVO4:Cr samples are the same [Supplementary Figure 15], indicating that Cr doping does not alter the chemical state of the CoOx cocatalyst[46]. Additionally, similar particle size and morphology of the deposited CoOx are identified for CoOx/FeVO4 and CoOx/
To reveal the origin of the extension in the light absorption edge and the enhancement of the charge separation, the density of states (DOS) was calculated. The crystal structure models of FeVO4 and FeVO4:Cr are given in Figure 4A and B, in which the latter case is constructed by replacing an Fe atom with a Cr atom. As shown in Figure 4C, the CBM and VBM of FeVO4 are mainly contributed by Fe 3d and O 2p orbitals, respectively. After Cr doping, an impurity level originating from the Cr 3d orbital is observed below the CBM [Figure 4D]. This impurity level induces a slight downshift of the CBM, leading to a decrease in the bandgap and facilitating the charge separation [Figure 4E].
CONCLUSIONS
In summary, it was demonstrated, for the first time, that the FeVO4 photocatalyst with an absorption edge of 575 nm could drive both photocatalytic water reduction and oxidation under visible light irradiation. After Cr doping, the corresponding photocatalytic water splitting activities could be efficiently promoted. Detailed analysis shows that the strategy of Cr doping can prolong the absorption edge and enhance the charge separation of the FeVO4 photocatalyst, contributing to improved photocatalytic water splitting performance. This work inaugurates a new application field of photocatalytic water splitting for FeVO4 semiconductors with a narrow bandgap, and identifies that Cr doping is an effective strategy to further promote the FeVO4 photocatalysts, both of which are expected to be extended to other photocatalytic reaction systems for efficient solar energy conversion.
DECLARATIONS
Authors’ contributions
Conception and design of the study: Chen, S.; Zhang, F.
Data collection and analysis: Wang, S.; Liu, C.
Sample preparation: Li, C.; Wang, N.; Li, C. Y.; Yuan, Z.
Paper writing and reviewing: Wang, S.; Liu, C.; Chen, S.; Zhang, F.
Availability of data and materials
Some results of supporting the study are presented in the Supplementary Materials. Other raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
We are grateful for the financial support from the National Natural Science Foundation of China (22272082, 21925206), the Fundamental Research Funds for the Central Universities, Nankai University (63213098), and the Foundation from Hebei Provincial Department of Science and Technology (226Z4307G).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Nishioka, S.; Osterloh, F. E.; Wang, X.; Mallouk, T. E.; Maeda, K. Photocatalytic water splitting. Nat. Rev. Methods. Primers. 2023, 3, 42.
2. Sun, K.; Qian, Y.; Jiang, H. L. Metal-organic frameworks for photocatalytic water splitting and CO2 reduction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217565.
3. Lin, L.; Yu, Z.; Wang, X. Crystalline carbon nitride semiconductors for photocatalytic water splitting. Angew. Chem. Int. Ed. Engl. 2019, 58, 6164-75.
4. Bai, Y.; Zhou, Y.; Zhang, J.; et al. Homophase junction for promoting spatial charge separation in photocatalytic water splitting. ACS. Catal. 2019, 9, 3242-52.
5. Takata, T.; Jiang, J.; Sakata, Y.; et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411-4.
6. Zhang, Y.; Zhao, J.; Wang, H.; et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56%. Nat. Commun. 2022, 13, 58.
7. Li, Y.; Peng, Y. K.; Hu, L.; et al. Photocatalytic water splitting by N-TiO2 on MgO (111) with exceptional quantum efficiencies at elevated temperatures. Nat. Commun. 2019, 10, 4421.
8. Lin, L.; Lin, Z.; Zhang, J.; et al. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649-55.
9. Wang, Y.; Huang, W.; Guo, S.; et al. Sulfur-deficient ZnIn2S4/oxygen-deficient WO3 hybrids with carbon layer bridges as a novel photothermal/photocatalytic integrated system for Z-scheme overall water splitting. Adv. Energy. Mater. 2021, 11, 2102452.
10. Dong, C.; Lu, S.; Yao, S.; et al. Colloidal synthesis of ultrathin monoclinic BiVO4 nanosheets for Z-scheme overall water splitting under visible light. ACS. Catal. 2018, 8, 8649-58.
11. Qi, Y.; Zhang, B.; Zhang, G.; et al. Efficient overall water splitting of a suspended photocatalyst boosted by metal-support interaction. Joule 2024, 8, 193-203.
12. Chen, S.; Ma, G.; Wang, Q.; et al. Metal selenide photocatalysts for visible-light-driven Z-scheme pure water splitting. J. Mater. Chem. A. 2019, 7, 7415-22.
13. He, Y.; Thorne, J.; Wu, C.; et al. What limits the performance of Ta3N5 for solar water splitting? Chem 2016, 1, 640-55.
14. Zhang, J.; Liu, K.; Zhang, B.; et al. Anisotropic charge migration on perovskite oxysulfide for boosting photocatalytic overall water splitting. J. Am. Chem. Soc. 2024, 146, 4068-77.
15. Jiang, L.; Yang, J.; Zhou, S.; et al. Strategies to extend near-infrared light harvest of polymer carbon nitride photocatalysts. Coord. Chem. Rev. 2021, 439, 213947.
16. Fujito, H.; Kunioku, H.; Kato, D.; et al. Layered perovskite oxychloride Bi4NbO8Cl: a stable visible light responsive photocatalyst for water splitting. J. Am. Chem. Soc. 2016, 138, 2082-5.
17. Lian, Z.; Sakamoto, M.; Kobayashi, Y.; et al. Anomalous photoinduced hole transport in type I core/mesoporous-shell nanocrystals for efficient photocatalytic H2 evolution. ACS. Nano. 2019, 13, 8356-63.
18. Wang, R.; He, H.; Shi, L.; et al. Unleashing photocarrier transport in mesoporous single-crystalline LaTiO2N for high-efficiency photocatalytic water splitting. Adv. Energy. Mater. 2024, 14, 2302996.
19. Ma, H.; Yang, X.; Tao, Z.; Liang, J.; Chen, J. Controllable synthesis and characterization of porous FeVO4 nanorods and nanoparticles. CrystEngComm 2011, 13, 897-901.
20. Zhao, Y.; Yao, K.; Cai, Q.; et al. Hydrothermal route to metastable phase FeVO4 ultrathin nanosheets with exposed {010} facets: synthesis, photocatalysis and gas-sensing. CrystEngComm 2014, 16, 270-6.
21. Sajid, M. M.; Zhai, H.; Shad, N. A.; et al. Photocatalytic performance of ferric vanadate (FeVO4) nanoparticles synthesized by hydrothermal method. Mater. Sci. Semicond. Process. 2021, 129, 105785.
22. Zhang, M.; Fang, Y.; Tay, Y. F.; et al. Nanostructured iron vanadate photoanodes with enhanced visible absorption and charge separation. ACS. Appl. Energy. Mater. 2022, 5, 3409-16.
23. Chen, H.; Zeng, J.; Chen, M.; et al. Improved visible light photocatalytic activity of mesoporous FeVO4 nanorods synthesized using a reactable ionic liquid. Chin. J. Catal. 2019, 40, 744-54.
24. Zhang, M.; Ma, Y.; Friedrich, D.; van de Krol, R.; Wong, L. H.; Abdi, F. F. Elucidation of the opto-electronic and photoelectrochemical properties of FeVO4 photoanodes for solar water oxidation. J. Mater. Chem. A. 2018, 6, 548-55.
25. Wang, W.; Zhang, Y.; Wang, L.; Bi, Y. Facile synthesis of Fe3+/Fe2+ self-doped nanoporous FeVO4 photoanodes for efficient solar water splitting. J. Mater. Chem. A. 2017, 5, 2478-82.
26. Alsulami, Q. A.; Rajeh, A.; Mannaa, M. A.; Albukhari, S. M.; Baamer, D. F. Preparation of highly efficient sunlight driven photodegradation of some organic pollutants and H2 evolution over rGO/FeVO4 nanocomposites. Int. J. Hydrog. Energy. 2021, 46, 27349-63.
27. Luangwanta, T.; Chachvalvutikul, A.; Kaowphong, S. Facile synthesis and enhanced photocatalytic activity of a novel FeVO4/Bi4O5Br2 heterojunction photocatalyst through step-scheme charge transfer mechanism. Colloid. Surface. A. 2021, 627, 127217.
28. Naqvi, S. Q.; Jennings, J. R.; Raza, S. A.; Soon, Y. W.; Liu, Y. Hole collection and surface kinetics in Mo-doped FeVO4 photoanodes during photoelectrochemical water oxidation. ACS. Appl. Energy. Mater. 2023, 6, 211-21.
29. Zeng, Q.; Fu, X.; Chang, S.; et al. Ordered Ti-doped FeVO4 nanoblock photoanode with improved charge properties for efficient solar water splitting. J. Colloid. Interface. Sci. 2021, 604, 562-7.
30. Zhang, M.; Pham, H. K.; Fang, Y.; Tay, Y. F.; Abdi, F. F.; Wong, L. H. The synergistic effect of cation mixing in mesoporous BixFe1-xVO4 heterojunction photoanodes for solar water splitting. J. Mater. Chem. A. 2019, 7, 14816-24.
31. Balu, S.; Chen, Y. L.; Chen, S. W.; Yang, T. C. K. Rational synthesis of BixFe1-xVO4 heterostructures impregnated sulfur-doped g-C3N4: a visible-light-driven type-II heterojunction photo(electro)catalyst for efficient photodegradation of roxarsone and photoelectrochemical OER reactions. Appl. Catal. B. Environ. 2022, 304, 120852.
32. Nguyen, T. H.; Zhang, M.; Septina, W.; et al. High throughput discovery of effective metal doping in FeVO4 for photoelectrochemical water splitting. Solar. RRL. 2020, 4, 2000437.
33. Dutta, D. P.; Ramakrishnan, M.; Roy, M.; Kumar, A. Effect of transition metal doping on the photocatalytic properties of FeVO4 nanoparticles. J. Photochem. Photobiol. A. 2017, 335, 102-11.
34. Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15-50.
35. Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999, 59, 1758.
36. Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865-8.
37. Anisimov, V. I.; Zaanen, J.; Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B. 1991, 44, 943.
38. Zhen, C.; Chen, X.; Chen, R.; et al. Liquid metal-embraced photoactive films for artificial photosynthesis. Nat. Commun. 2024, 15, 1672.
39. Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Cryst. A. 1976, 32, 751-67.
40. Thalluri, S. M.; Martinez, S. C.; Hussain, M.; et al. Evaluation of the parameters affecting the visible-light-induced photocatalytic activity of monoclinic BiVO4 for water oxidation. Ind. Eng. Chem. Res. 2013, 52, 17414-8.
41. Brown, I. D.; Wu, K. K. Empirical parameters for calculating cation–oxygen bond valences. Acta. Crystallogr. B. Struct. Sci. 1976, 32, 1957-9.
42. Wu, Y.; Tao, X.; Qing, Y.; et al. Cr-doped FeNi-P nanoparticles encapsulated into N-doped carbon nanotube as a robust bifunctional catalyst for efficient overall water splitting. Adv. Mater. 2019, 31, 1900178.
43. Marshall-Roth, T.; Libretto, N. J.; Wrobel, A. T.; et al. A pyridinic Fe-N4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat. Commun. 2020, 11, 5283.
44. Pei, H.; Peng, L.; Jiang, Z.; Zhang, Y.; Li, R.; Peng, T. Gradient-tuned VO4 vacancies in BiVO4 photoanode for boosting bulk hole transport and oxygen evolution reaction performance. Adv. Funct. Mater. 2024, 34, 2401122.
45. Wang, S.; He, T.; Chen, P.; et al. In situ formation of oxygen vacancies achieving near-complete charge separation in planar BiVO4 photoanodes. Adv. Mater. 2020, 32, 2001385.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Author Biographies








Data & Comments
Data





















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].