Human exposure assessment of organophosphate esters (OPEs) through dust ingestion and dermal absorption in Colombian cities
Abstract
Concentrations of 19 organophosphate esters (OPEs) were determined in dust samples collected from house and car indoor microenvironments in three Colombian cities. ∑OPE concentrations ranged from 1.31 to 599 μg/g. Mean concentrations of dust homes were 82.6, 48.3, and 46.7 μg/g for Cartagena, Bogotá, and Medellín, respectively. The pollution inside cars was somewhat higher than in houses, with a mean value of 231 μg/g. Sixteen compounds were detected, being TPHP, DCP, TEP, and TCEP the most frequently detected. As for OPEs with higher levels in houses, we found (mean ± SD) 35.2 ± 37.1 μg/g for TDCIPP in Cartagena, 35.6 ± 80.2 μg/g for TPHP in Cartagena, 15.9 ± 31.4 μg/g for DCP in Cartagena, 35.7 ± 19.1 μg/g for TBOEP in Bogotá, 15.7 ± 14.8 μg/g for 4IPPDPP in Medellín, and 17.5 ± 22.9 μg/g for TCEP in Cartagena, while the highest OPE value found in cars was 176 ±
Keywords
INTRODUCTION
Flame retardants (FRs) are chemical additives that are incorporated into different materials such as plastics, textiles, foams, furniture, and electronic materials[1,2]. Their function is to prevent combustion or reduce the spread of fire after ignition[2]. Organophosphate esters (OPEs) have come to the fore in recent years due to their increasing use as an alternative to legacy brominated FRs[3-5]. Global demand for OPEs increased from 500,000 tons in 2011 to 680,000 tons in 2015[6,7]. OPEs are also widely used as plasticizers in furniture, textile coatings, upholstery, electronics, paints, polyurethane foams, lubricants, and hydraulic fluids[8].
OPEs have been found in different environmental matrices such as wastewater[9], surface water[10], drinking water[10,11], air[3], and indoor dust[12], as well as in biotic samples such as river fish[13] and marine mammals[14]. In addition, toxicology and epidemiology studies indicate that these compounds are associated with immunotoxicity[15], cardiotoxicity[16], neurotoxicity[17], adverse reproductive effects, and endocrine disruption[18], and they are carcinogenic[19]. Tri-n-butyl phosphate (TNBP) causes sick building syndrome, which is related to the effects produced by chemical, physical, biological, and ergonomic agents, often related to the structure, distribution, facilities, and equipment of the building, as described by the World Health Organization[20]. OPEs are also considered to have neurodevelopmental problems after long-term exposure[21]. There is evidence that tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) causes adverse effects on thyroid hormone (TH) imbalance in aquatic and avian organisms[22] and thyroid function and hormone homeostasis in mammals[23]. Tris-2-chloroethyl phosphate (TCEP) exhibits carcinogenicity and was eliminated by the EU in 1980[24,25]. Furthermore, TDClPP may be carcinogenic[19].
Humans spend their lives in a variety of indoor microenvironments, such as homes, offices, and cars. Research reports that OPEs are widely used in various industrial products such as plastics, building materials, textiles, furniture, electronic parts, and vehicle parts[26]. OPEs can be released from a multitude of products, which inevitably lead to them being found in these indoor microenvironments. Indoor environments are considered a hotspot for human exposure to OPEs[27], which can occur from several routes including air inhalation, dust ingestion, dust dermal absorption, and dietary intake[5,28]. However, the pathways related with dust and air have been identified as the most important matrices for estimation of non-dietary exposure in the indoor microenvironment[26,28], probably because of the large surface area in dust, becoming important deposits of OPEs[5,29]. In general, OPE levels in indoor dust are significantly higher than those in outdoor dust[30,31]. Total OPE levels have been reported in indoor dust where concentrations were generally at the nanogram per gram level, but in some cases they reach values at the microgram per gram level[12,32].
This study aimed to evaluate the presence of different OPEs in dust samples from microenvironments of houses and cars, collected from the three most populated, industrialized, and polluted cities (Bogotá, Medellín, and Cartagena) in Colombia. In addition, we assessed the dust pathway as non-dietary human exposure to these compounds through ingestion and dermal routes for several age groups (infants, toddlers, children, teenagers, and adults) as recommended by US-EPA[33-35]. To the best of our knowledge, this is the first study to show the occurrence of these FRs and plasticizers in several cities in Colombia.
EXPERIMENTAL
Sampling collection
Dust samples were collected in three Colombian cities from house and car interiors in the cities of Bogotá (n = 20 houses), Medellín (n = 15 houses and n = 5 cars), and Cartagena (n = 20 houses) in 2018.
These cities have different climatic characteristics. Bogotá is the capital and the largest city in Colombia, located in the mountains of the Andes at 2586 m above sea level (asl), with moderately cold temperatures (an average of 14 °C) that fall below 5 °C in the rainy season. The relative humidity varies between 77% and 83%. Medellín is the second-largest city in Colombia, located in the Aburra Valley in the Andes at 1495 m asl, with humid weather (subtropical relative humidity between 63% and 73%) and moderate temperatures of 21.6 °C on average. Cartagena is a coastal city at sea level on the shores of the Caribbean Sea, humid (on average 78%-82%) with temperatures varying from 24 to 31 °C but generally higher than those cities located in the Andes.
Dust samples were collected from single or multiple bedrooms and living rooms of homes and apartments, selected for sampling. Floor dust samples were obtained from vacuum cleaner bags in each of the sampling sites. Samples were taken at the room normal conditions to observe the current human exposure. Prior to the analysis, samples were manually cleaned, removing particulate matter from stones, plastic, iron, grains, wood, and shavings. Samples were packaged in glass bottles, previously sterilized, labeled. and free of any organic or inorganic contamination, and immediately stored at -18 °C.
Standards and reagents
Analytical standards were acquired from different companies. Tris(2-butoxyethyl) phosphate (TBOEP), tri(2-chloroethyl) phosphate (TCEP), tris(chloroisopropyl) phosphate (TCIPP), trihexyl phosphate (THP) and tris(2-ethylhexyl) phosphate (TEHP) were obtained from Santa Cruz Biotechnology (SantaCruz, CA, USA); isodecyldiphenyl phosphate (IDPP) and 2-ethylhexyldiphenyl phosphate (EHDPP) were acquired by AccuStandard (New Haven, CT, USA); diphenylcresyl phosphate (DCP), tri(n-butyl)phosphate (TNBP), triphenyl phosphate (TPHP), triphenylphosphine oxide (TPPO), tris(1,3-dichloro-2-propyl) phosphate (TDClPP), triethyl phosphate (TEP), and tri-n-propyl phosphate (TPP) were acquired from Sigma-Aldrich (St. Louis, MO, USA); tricresyl phosphate (TMCP) was acquired from Dr. Ehrenstorfer (Augsburg, Germany); 2-isopropylphenyl diphenyl phosphate (2IPPDPP), 4-isopropylphenyl diphenyl phosphate (4IPPDPP), and bis(4-isopropylphenyl) phenyl phosphate (B4IPPPP) were acquired from Wellington Laboratories Inc. (Guelph, ON, Canada); and tris(2-isopropylphenyl) phosphate (T2IPPP) was purchased from Chiron (Trondheim, Norway). The isotopically labeled compounds d15-TDClPP, d27-TNBP,
Sample preparation
Throughout all analysis processes, plastic material was avoided due to potential contamination, as our analytes are used as plasticizers. We tried to minimize as much as possible blank signals, i.e., heating all the non-volumetric material at 340 °C and rinsing with ethanol and hexane:acetone (1:1) just before use. For each batch of samples, a blank was included. Blank levels were subtracted from corresponding samples.
The analytical method applied was previously developed[13]. The dust sample (between 0.5 and 1 g) was spiked with internal standards (100 ng of d12-TCEP, d15-TDClPP, d27-TNBP, d15-TPHP, and 13C2-TBOEP) and loaded into a 22 mL extraction cell previously filled with 0.5 g of powdered copper (particle size <
Instrumental analysis
An online sample purification and analysis method based on turbulent flow chromatography (TFC) (Thermo Scientific TurboFlow™ system) in combination with a triple quadrupole (QqQ) tandem mass spectrometry (MS-MS) and a heated-electrospray ionization source (H-ESI) was used. Two liquid chromatography (LC) quaternary pumps and three LC columns were employed: Cyclone™-P (0.5 mm ×
Selective reaction monitoring mode was used for all compounds with two transitions monitored for each analyte. The most intense transition was used for quantification, while the second provided confirmation. Instrumental working parameters such as retention times, transitions, declustering potential, and collision energies are summarized in Supplementary Table 2.
Confirmation criteria for the detection and quantification of OPEs should include the following: (1) retention time for all transition monitored for a given analyte should maximize simultaneously ± 1 s, with signal-to-noise ratio ≥ 3 for each; and (2) the ratio between the two monitored transitions should be within 15% of the theoretical. Quantification was carried out by internal standard method based on the use of labeled OPE standards with d15-TDClPP, d27-TNBP, d12-TCEP, 13C12-TBOEP, and d15-TPHP as internal standards.
Analytical parameters such as recoveries, limits of detection (LODs), and limits of quantification (LOQs) are summarized in Supplementary Table 3. These quality parameters were calculated by analyzing four replicates of spiked samples. Samples were spiked with 10 ng of OPE analytes and extraction was performed following the same procedure as for regular dust samples. Previously to final reconstitution, 10 ng of labeled internal standards of OPEs were spiked. Our analytical methodology provided recoveries ranging between 59% and 118%, LODs between 0.001 and 0.042 μg/g, and LOQs between 0.003 and 0.141 μg/g.
Statistical analysis
For the statistical analysis, a logarithmic transformation was performed on the data, which facilitated their use as some values obtained exceed the mean obtained by several orders of magnitude. Statistical analyses were carried out with R statistical software environment and Statgraphics Centurion XVIII, together with Excel. Significant statistical differences were considered when the P-value was ≤ 0.05.
Human risk assessment
Previous studies reported that humans can be exposed to OPEs in dust mainly via dust ingestion and dermal absorption. To evaluate human exposure, estimated daily intakes (EDIs) were calculated, expressed in μg/kg body weight (bw)/day, using Equations (1)-(3):
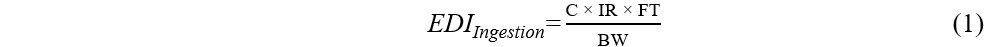
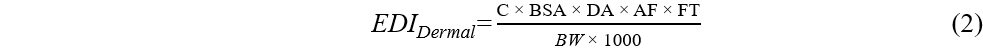
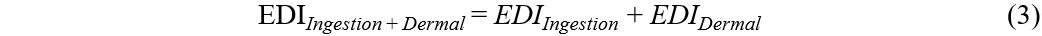
where C (μg/g) represents the mean OPE concentration in dust samples, IR (g/day) is the daily dust ingestion rate, FT (%) is the fraction of time spent at the microenvironment (home or car), BW (kg) is the body weight, BSA (cm2/day) is the body surface area, DA (mg/cm2) is the dust adhered to the skin, and AF is the fraction of OPEs absorbed by the skin[36-39].
The hazard quotient (HQ) for non-carcinogens was estimated for OPEs using Equation (4):
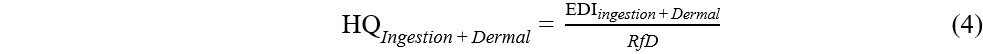
where RfD (ng/kg/day) is the reference dose for OPEs. RfD values were only available for TCEP, TPPO, TCIPP, TDCIPP, TMCP, and TEHP[37,40]. OPEs pose a health risk if the estimated HQs were greater than 1 (HQ > 1).
Lifetime average daily dose (LADD) and incremental lifetime cancer risk (ILCR) were estimated for OPE carcinogens exposure via dust thought dermal and ingestion pathways using Equations (5)-(7). The exposure assessment was performed for five age groups: infants (< 1 year), toddlers (1-5 years), children (6-11 years), teenagers (12-19 years), and adults (≥ 20 years).
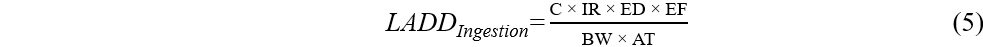
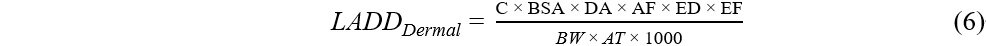
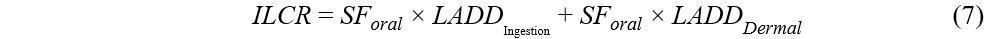
where ED (year) is the exposure duration, EF (day/year) is the exposure frequency, AT (day) is the average time, and SF (mg/kg/day)-1 is the slope factor for cancer risk. SF values were only available for the oral route for TCEP and TEHP[41-43]. SF values were not available for dermal route, and therefore oral SF values were used for all routes. Exposure to OPEs (TCEP and TEHP) through dust ingestion and dermal absorption was considered as cancer risk when the estimated ILCR was ≥ 1 × 10-6[41-44].
Non-cancer and cancer endpoints, RfD, and SF of target OPE compounds are collected in Supplementary Table 4.
Probabilistic model
The estimated EDI, HQ, LADD, and ILCR values on the health risk exposure of OPEs are represented as single values. The input values for calculation may contain some degree of uncertainty arising from multiple and different sources. Thus, simple point estimations are inaccurate. Therefore, an uncertainty analysis was performed associated relative errors of the factors through Monte Carlo simulation (MCS). The probabilistic analysis of MCS allows estimating the uncertainties of all possible outcomes and assessing the impact of risk, and therefore better decisions can be made under uncertainty conditions. In this study, MCS was performed using The Crystal Ball software, version 11.1.2.4 (Oracle, Inc. Redwood. US), and the number of simulations is defined as 10,000. A probability distribution of the exposure factors was assumed, and the detailed description of abbreviations, sources, probability distribution, and units for C, IR, FT, BW, BSA, DA, AF, FT, ED, EF, AT, RfD, and SF are available at Supplementary Tables 4 and 5. The uncertainty analysis for EDI, HQ, LADD, and ILCR was performed at 10,000 random repetitions for all calculations.
RESULTS AND DISCUSSION
OPE levels in indoor dust
The mean concentrations of OPEs in dust samples collected from different homes and cars in Colombian cities are summarized in Table 1 (for individual sample results, see Supplementary Table 6). OPEs were detected in all analyzed samples, indicating widespread contamination by these emerging pollutants, with levels ranging between 1.31 and 599 μg/g. ∑OPE concentrations at homes varied between cities, being the highest values those obtained from Cartagena (mean ± SD), 82.6 ± 113 μg/g, followed by Bogotá and
Concentrations (μg/g), range, and detection frequency of OPEs in dust indoor samples from Colombian cities
| OPEs | Bogotá | Cartagena | Medellín | |||||||||
| Homes | Homes | Homes | Cars | |||||||||
| Mean ± SD* | Median (range) | DF (%) | Mean ± SD | Median (range) | DF (%) | Mean ± SD | Median (range) | DF (%) | Mean ± SD | Median (range) | DF (%) | |
| TEP | 1.94 ± 1.82 | 1.02 (0.14-6.75) | 55 | 3.15 ± 7.1 | 1.0 (0.14-31.9) | 84 | 3.28 ± 4.2 | 1.8 (0.34-16.2) | 79 | 3.43 ± 3.5 | 2.7 (0.11-9.32) | 100 |
| TCEP | 7.97 ± 7.5 | 5.19 (0.31-51.9) | 55 | 17.5 ± 22.9 | 7.1 (1.32-97.1) | 74 | 9.12 ± 14.3 | 1.61 (0.24-54.0) | 50 | 13.8 ± 8.7 | 16.5 (6.83-18.1) | 60 |
| TPPO | 0.61 ± 0.42 | 0.62 (0.24-1.60) | 50 | 0.89 ± 0.8 | 0.5 (0.52-1.74) | 32 | 9.13 ± 19.8 | 1.0 (0.30-74.5) | 64 | 1.33 ± 0.7 | 1.3 (1.27-1.40) | 60 |
| TCIPP | 4.56 ± 2.7 | 2.98(1.30-11.0) | 25 | 5.7 ± 3.0 | 6.9 (0.50-11.0) | 32 | 2.66 ± 1.9 | 2.5 (0.45-6.16) | 43 | 71.1 ± 60.4 | 65.9 (3.61-134) | 60 |
| TPP | 0.52 ± 0.30 | 0.67 (0.28-0.76) | 45 | 0.62 ± 0.3 | 0.7 (0.36-0.73) | 21 | 0.62 ± 0.34 | 0.71 (0.35-0.76) | 50 | 0.67 ± 0.4 | 0.7 (0.61-0.72) | 60 |
| TDCIPP | 0.96 ± 0.64 | 0.30 (0.11-2.22) | 30 | 35.2 ± 37.1 | 4.5 (0.29-122) | 37 | 1.24 ± 0.86 | 0.81 (0.18-2.80) | 36 | 176 ± 144 | 176 (24.7-328) | 40 |
| TPHP | 21.1 ± 18.4 | 18.3 (1.26-70.2) | 90 | 35.6 ± 80.2 | 8.9 (0.43-325) | 100 | 20.5 ± 15 | 25.6 (0.75-36.5) | 64 | 126 ± 155 | 19.7 (4.65-352) | 60 |
| DCP | 4.75 ± 5.0 | 3.64 (0.21-17.4) | 95 | 15.9 ± 31.4 | 2.6 (0.40-121) | 74 | 5.57 ± 5.32 | 2.91 (0.87-17.6) | 71 | 2.64 ± 1.6 | 2.8 (0.88-4.04) | 80 |
| TBOEP | 35.7 ± 19.1 | 33.9 (10.4-61.0) | 30 | 5.85 ± 3.7 | 4.8 (0.46-14.4) | 32 | 1.37 ± 0.82 | 1.7 (0.03-1.87) | 43 | 34.1 ± 37.1 | 12.5 (3.66-86.1) | 60 |
| 2IPPDPP | 0.25 ± 0.17 | 0.64 (0.09-0.47) | 60 | 0.24 ± 0.1 | 0.3 (0.22-0.25) | 16 | 0.49 ± 0.5 | 0.32 (0.11-1.92) | 57 | 0.27 ± 0.16 | 0.23 (0.22-0.36) | 60 |
| 4IPPDPP | 7.34 ±7.03 | 4.09 (0.06-30.2) | 40 | 7.51 ± 4.3 | 4.4 (3.41-18.0) | 21 | 15.7 ± 14.8 | 8.7 (3.22-55.8) | 43 | 7.53 ± 6.7 | 4.02 (2.33-16.2) | 60 |
| TMCP | 1.16 ± 1.04 | 0.35 (0.17-4.68) | 25 | 3.83 ± 2.4 | 2.7 (0.42-8.21) | 26 | 2.29 ± 2.0 | 1.53 (0.21-7.43) | 43 | 0.98 ± 0.58 | 0.90 (0.70-1.34) | 60 |
| EHDPP | 0.28 ± 0.15 | 0.26 (0.11-0.59) | 25 | 0.1 ± 0.05 | 0.1 (0.11-0.14) | 16 | 0.14 ± 0.07 | 0.14 (0.12 -0.18) | 43 | 0.22 ± 0.13 | 0.21 (0.17-0.29) | 60 |
| B4IPPPP | 0.25 ± 7.03 | 4.09 (0.02-0.48) | 10 | nd | nd | 0 | 5.16 ± 2.75 | 5.19 (0.07-10.3) | 14 | 0.17 ± 0.08 | 0.17 (0.17-10.3) | 20 |
| T2IPPP | 1.75 ±1.59 | 1.05 (0.40-6.08) | 45 | 1.76 ± 0.7 | 1.7 (1.26-2.35) | 16 | 9.11 ± 15.8 | 2.02 (0.51-60.2) | 57 | 1.92 ± 1.18 | 1.49 (1.46-2.81) | 60 |
| TEHP | 5.24 ± 1.17 | 5.24 (nd-5.24) | 5 | nd | nd | 0 | nd | nd | 0 | 0.64 ± 0.42 | 0.64 (0.32-0.96) | 40 |
| ∑OPEs | 48.3 ± 34.3 | 38.3 (1.31-121) | 100 | 82.6 ± 113 | 39.2 (1.7-447) | 88 | 46.7 ± 48.1 | 34.3 (1.58-368) | 94 | 231 ± 247 | 122 (21.7-599) | 100 |
Different studies have been conducted on OPE occurrence in dust samples [Supplementary Table 7]. Comparison with published data must be carried out with caution because analytical methodologies included different OPEs (3-20 compounds) as well as differences in the sampling methodology. A study on indoor dust from homes in 12 different countries (China, Colombia, USA, India, Japan, and Vietnam, among others) reported values of 0.05-249 μg/g[12]. A similar range of concentrations has been observed in dust from homes in USA[45], ranging from 16 to 224 μg/g. The most recent research[46] studied OPE levels in dust from homes, offices, and schools, obtaining levels between 5.82 and 148 μg/g. The values reported in these studies are very close to those found in our research, where we reported values between 1.31 and
OPE patterns
Sixteen of nineteen analyzed compounds were detected in dust samples and three were not detected in any samples (TNBP, IDPP, and THP). TPHP was the compound with the highest frequency detection in home dust samples (87%), followed by DCP (81%), TEP (72%), and TCEP (60%). In the case of car dust, TEP was detected in all analyzed samples (100%), followed by DCP (80%), and TPHP and TCEP were detected in the 60% of analyzed samples.
The persistence of TPHP in dust samples could be related to their widespread use in polyvinyl chloride (PVC)-based products, plastics, flooring, electronic products, hydraulic fluids, and flexible polyurethane foam for car upholstery[48]. Zhou and Püttmann[49] also reported a high TPHP occurrence (93%) in household samples and building materials markets. A study also related high TPHP values in dust to its presence in mattresses[50]. The high frequency detection of DCP could be related to their common use as plasticizer for PVC[20]. TEP follows as the most frequently detected compound, and this OPE is used in resins, plastics, rubbers, defoamers, and as flame retardant in rigid urethane foam[51]. Regarding TCEP, this compound is being replaced by TCIPP due to its carcinogenicity[52], and, consequently, we also observed a high contribution of TCIPP in the total concentration of dust samples.
Regarding concentration levels, the most contributing compound on the total OPE concentrations in home dust was TCIPP (mean value of 81.7 μg/g), followed by TPHP (27.0 μg/g), TDClPP and TBOEP (14.3 μg/g), and TCEP (13.7 μg/g). For car dust samples, TDClPP was the most contributing OPE (mean value of
Figure 1. Percentage contribution of detected OPEs to the total concentration levels in dust from homes of Cartagena, Bogotá, and Medellín, as well as in Medellín cars. OPEs: Organophosphate esters; TEP: triethyl phosphate; TCEP: tri(2-chloroethyl) phosphate; TPPO: triphenylphosphine oxide; TCIPP: tris(chloroisopropyl) phosphate; TPP: tri-n-propyl phosphate; TDClPP: tris(1,3-dichloro-2-propyl) phosphate; TPHP: triphenyl phosphate; DCP: diphenylcresyl phosphate; TBOEP: tris(2-butoxyethyl) phosphate; 2IPPDPP: 2-isopropylphenyl diphenyl phosphate; 4IPPDPP: 4-isopropylphenyl diphenyl phosphate; TMCP: tricresyl phosphate; EHDPP: 2-ethylhexyldiphenyl phosphate; B4IPPP: bis(4-isopropylphenyl) phenyl phosphate; IPPP: tris(2-isopropylphenyl) phosphate; TEHP: tris(2-ethylhexyl) phosphate.
Zhou et al.[32] analyzed indoor dust samples from different indoor microenvironments in Germany, and they found that TBOEP and TCIPP were the most abundant OPEs. Another study of indoor dust showed that the most abundant OPEs were TCIPP, TCEP, and TPHP, with mean values of 0.52, 0.29, and
Daily intake estimations
EDI values were calculated based on exposure factors and OPE concentrations in dust from homes. Humans can be exposed to OPEs by dust through non-dietary routes such as ingestion, air inhalation, and dermal absorption. However, ingestion and dermal absorption have been reported as predominant pathways[12]. The EDIs of OPEs through dust ingestion (EDIIngestion) and dermal absorption (EDIDermal) were estimated. The results are presented in Supplementary Tables 8 and 9. Because body weight, ingestion rate, and body surface area vary with the age, we estimated the EDIs for five age groups: infants (< 1 year), toddlers (1-3 years), children (4-10 years), teenagers (11-18 years), and adults (≥ 19 years).
The obtained mean EDIIngestion values ranged from 0.03 ng TPPO/kg-bw/day for teenagers and adults from Bogotá to 110 ng TDClPP/kg-bw/day for toddlers from Cartagena. Regarding EDIDermal, mean values were between 0.49 ng TPPO/kg-bw/day for adults from Bogotá and 42.7 ng TCEP/kg-bw/day for infants from Cartagena. EDIs varied depending on the pathway, the age group, and the city. Among the cities, EDIs decreased for the most of OPEs in the following order: Cartagena > Medellín > Bogotá. As regards the age group, in general, EDIs decreased with the age. Toddlers were the most exposed group for dust ingestion, being their EDIIngestion values on average 50 and 70 times higher than those for teenagers and adults, respectively. Dust ingestion is an important pathway of pollutant exposure for toddlers because they spend a significant amount of time on the floor, where they touch different objects with their fingers and transfer them to the mouth, as a result of the hand-to-mouth behavior. On the other hand, infants presented the highest EDIDermal values, being 50 times greater than those for teenagers and adults. Moreover, dust ingestion was the dominant pathway for infants and toddlers, whereas dermal absorption was the dominant pathway for teenagers and adults [Figure 2]. In the case of Medellín, where we collected dust samples from both houses and cars, we estimated the EDI values from the exposure in both microenvironments. The EDIHouse + Car values were estimated as the sum of the EDIHouse and EDICar from dust ingestion and dermal absorption [Supplementary Table 10]. EDIHouse + Car values ranged from 0.05 ng TEHP/kg-bw/day for teenagers to 51.5 ng TDClPP/kg-bw/day for infants. In general, EDIHouse + Car via dust decreased in the order: toddlers > infants > children > teenagers > adults. Moreover, the EDIHouse + Car values of OPEs for toddlers decreased in the order: TCEP > TDClPP > TPPO > TCIPP > TMCP > TEHP. For both routes of exposure, dust ingestion and dermal absorption, EDICar values were slightly different from EDIHouse ones. Variations were related to the different OPE profiles in cars and homes as well as the different fraction time of the day spent traveling in a car (0.044-0.065, depending on the age group) and at home (0.792-0.875). Few studies have focused on the OPE exposure traveling in a car or other type of transport. Our obtained EDI values for toddlers (34.6 and 0.24 ng/kg-bw/day for TCIPP and TEHP, respectively) were similar to those reported in Thailand (22.4 and 0.16 ng/kg-bw/day for TCIPP and TEHP, respectively)[55].
Figure 2. Contribution of different pathways (dust ingestion and dermal absorption) to OPE exposure via home dust, for different age groups in Bogotá. TCEP: Tri(2-chloroethyl) phosphate; TPPO: triphenylphosphine oxide; TCIPP: tris(chloroisopropyl) phosphate; TDCIPP: tris(1,3-dichloro-2-propyl) phosphate; TMCP: tricresyl phosphate; TEHP: tris(2-ethylhexyl) phosphate.
Human exposure assessment
Monte Carlo analysis has become a powerful tool in environmental risk assessment because it incorporates the quantitative uncertainty analysis into the risk estimate. HQ values were simulated by Monte Carlo technique providing a useful, systematic, and quantitative description of the human health risk of OPE exposure via dust ingestion and dermal absorption. The final result was a probabilistic density function (PDF) of the HQ for OPEs exposure through dust. HQ values were reported for the sum of the ingestion and dermal absorption pathways, which varied depending on the age group, city, and type of OPE [Table 2, Figure 3]. The obtained mean values ranged from 0 to 0.014, being always below the safe value of 1. Similar results were obtained when exposure to dust car was also included and added to the exposure to dust homes [Supplementary Table 11]. The highest HQ values were found for TCEP: this compound was one of the most frequently detected OPEs (60%), found at high concentration levels in home dust (mean value of
Figure 3. Mean hazard quotients (HQs) simulated by MCS for OPE exposure via home dust: (A) HQ variation among cities; and (B) HQ variation by age group. MCS: Monte Carlo simulation; OPE: organophosphate ester; TCEP: tri(2-chloroethyl) phosphate; TCIPP: tris(chloroisopropyl) phosphate; TDCIPP: tris(1,3-dichloro-2-propyl) phosphate; TEHP: tris(2-ethylhexyl) phosphate; TMCP: tricresyl phosphate; TPPO: triphenylphosphine oxide.
Hazard quotients of OPEs via dust ingestion and dermal absorption exposure
| OPEs | Age groups | Bogotá | Cartagena | Medellín | |||||||||
| Mean | SD | Median | 95th percentile | Mean | SD | Median | 95th percentile | Mean | SD | Median | 95th percentile | ||
| TCEP | Infant | 5.52E-03 | 6.65E-03 | 3.63E-03 | 1.61E-02 | 1.24E-02 | 1.82E-02 | 6.70E-03 | 4.14E-02 | 6.12E-03 | 1.01E-02 | 3.06E-03 | 2.16E-02 |
| Toddler | 6.13E-03 | 7.88E-03 | 3.97E-03 | 1.80E-02 | 1.36E-02 | 2.09E-02 | 7.30E-03 | 4.55E-02 | 5.91E-03 | 9.72E-03 | 2.92E-03 | 2.08E-02 | |
| Children | 3.62E-03 | 4.20E-03 | 2.40E-03 | 1.04E-02 | 8.09E-03 | 1.18E-02 | 4.41E-03 | 2.66E-02 | 4.13E-03 | 7.43E-03 | 2.04E-03 | 1.44E-02 | |
| Teenager | 2.08E-03 | 2.20E-03 | 1.48E-03 | 5.74E-03 | 4.64E-03 | 6.35E-03 | 2.72E-03 | 1.49E-02 | 2.33E-03 | 3.68E-03 | 1.24E-03 | 7.90E-03 | |
| Adult | 1.94E-03 | 2.07E-03 | 1.37E-03 | 5.27E-03 | 4.28E-03 | 5.55E-03 | 2.52E-03 | 1.38E-02 | 2.16E-03 | 3.33E-03 | 1.16E-03 | 7.07E-03 | |
| TPPO | Infant | 1.09E-04 | 1.17E-04 | 7.51E-05 | 3.01E-04 | 1.59E-04 | 1.46E-04 | 1.19E-04 | 4.12E-04 | 1.74E-03 | 4.80E-03 | 6.14E-04 | 6.22E-03 |
| Toddler | 1.28E-04 | 1.42E-04 | 8.72E-05 | 3.70E-04 | 1.88E-04 | 1.79E-04 | 1.36E-04 | 5.11E-04 | 1.70E-03 | 4.70E-03 | 5.98E-04 | 6.09E-03 | |
| Children | 6.64E-05 | 6.98E-05 | 4.62E-05 | 1.81E-04 | 9.78E-05 | 9.16E-05 | 7.20E-05 | 2.53E-04 | 1.05E-03 | 2.65E-03 | 3.77E-04 | 3.83E-03 | |
| Teenager | 2.78E-05 | 2.14E-05 | 2.19E-05 | 6.76E-05 | 4.09E-05 | 2.59E-05 | 3.46E-05 | 8.98E-05 | 4.34E-04 | 9.53E-04 | 1.77E-04 | 1.54E-03 | |
| Adult | 2.56E-05 | 1.92E-05 | 2.05E-05 | 6.19E-05 | 3.79E-05 | 2.37E-05 | 3.21E-05 | 8.26E-05 | 4.07E-04 | 8.99E-04 | 1.65E-04 | 1.44E-03 | |
| TCIPP | Infant | 2.05E-03 | 1.68E-03 | 1.58E-03 | 5.17E-03 | 2.60E-03 | 2.10E-03 | 2.04E-03 | 6.30E-03 | 1.20E-03 | 1.18E-03 | 8.73E-04 | 3.14E-03 |
| Toddler | 2.33E-03 | 2.11E-03 | 1.75E-03 | 6.11E-03 | 2.93E-03 | 2.44E-03 | 2.26E-03 | 7.34E-03 | 1.16E-03 | 1.15E-03 | 8.35E-04 | 3.05E-03 | |
| Children | 1.35E-03 | 1.19E-03 | 1.03E-03 | 3.47E-03 | 1.69E-03 | 1.35E-03 | 1.35E-03 | 3.97E-03 | 7.78E-04 | 7.33E-04 | 5.75E-04 | 2.00E-03 | |
| Teenager | 7.33E-04 | 5.05E-04 | 6.08E-04 | 1.67E-03 | 9.19E-04 | 5.44E-04 | 7.85E-04 | 1.94E-03 | 4.23E-04 | 3.19E-04 | 3.37E-04 | 1.04E-03 | |
| Adult | 6.78E-04 | 4.53E-04 | 5.63E-04 | 1.54E-03 | 8.52E-04 | 4.94E-04 | 7.36E-04 | 1.79E-03 | 3.92E-04 | 2.95E-04 | 3.14E-04 | 9.69E-04 | |
| TDCIPP | Infant | 1.74E-04 | 1.79E-04 | 1.21E-04 | 4.84E-04 | 6.40E-03 | 9.13E-03 | 3.75E-03 | 2.04E-02 | 2.24E-04 | 2.56E-04 | 1.53E-04 | 6.19E-04 |
| Toddler | 2.03E-04 | 2.08E-04 | 1.40E-04 | 5.63E-04 | 7.42E-03 | 1.04E-02 | 4.31E-03 | 2.41E-02 | 2.19E-04 | 2.52E-04 | 1.49E-04 | 6.05E-04 | |
| Children | 1.06E-04 | 1.08E-04 | 7.46E-05 | 2.92E-04 | 3.91E-03 | 5.61E-03 | 2.28E-03 | 1.22E-02 | 1.37E-04 | 1.53E-04 | 9.52E-05 | 3.82E-04 | |
| Teenager | 4.40E-05 | 3.17E-05 | 3.57E-05 | 1.03E-04 | 1.63E-03 | 1.83E-03 | 1.08E-03 | 4.82E-03 | 5.69E-05 | 4.53E-05 | 4.48E-05 | 1.40E-04 | |
| Adult | 4.09E-05 | 3.00E-05 | 3.30E-05 | 9.71E-05 | 1.51E-03 | 1.68E-03 | 9.88E-04 | 4.42E-03 | 5.28E-05 | 4.15E-05 | 4.17E-05 | 1.26E-04 | |
| TMCP | Infant | 2.10E-04 | 2.76E-04 | 1.32E-04 | 6.41E-04 | 6.92E-04 | 6.86E-04 | 5.02E-04 | 1.85E-03 | 4.13E-04 | 4.87E-04 | 2.67E-04 | 1.24E-03 |
| Toddler | 2.47E-04 | 3.20E-04 | 1.52E-04 | 7.52E-04 | 8.11E-04 | 7.98E-04 | 5.72E-04 | 2.23E-03 | 4.04E-04 | 4.79E-04 | 2.60E-04 | 1.22E-03 | |
| Children | 1.29E-04 | 1.69E-04 | 8.11E-05 | 3.87E-04 | 4.25E-04 | 4.08E-04 | 3.05E-04 | 1.16E-03 | 2.58E-04 | 3.31E-04 | 1.64E-04 | 7.47E-04 | |
| Teenager | 5.36E-05 | 5.27E-05 | 3.86E-05 | 1.46E-04 | 1.78E-04 | 1.25E-04 | 1.45E-04 | 4.14E-04 | 1.06E-04 | 9.90E-05 | 7.75E-05 | 2.81E-04 | |
| Adult | 4.94E-05 | 4.66E-05 | 3.56E-05 | 1.33E-04 | 1.64E-04 | 1.14E-04 | 1.34E-04 | 3.81E-04 | 9.87E-05 | 9.04E-05 | 7.29E-05 | 2.64E-04 | |
| TEHP | Infant | 1.91E-04 | 1.40E-04 | 1.53E-04 | 4.44E-04 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 |
| Toddler | 2.24E-04 | 1.66E-04 | 1.77E-04 | 5.27E-04 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
| Children | 1.16E-04 | 8.32E-05 | 9.46E-05 | 2.58E-04 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
| Teenager | 4.86E-05 | 1.74E-05 | 4.55E-05 | 8.05E-05 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
| Adult | 4.50E-05 | 1.57E-05 | 4.23E-05 | 7.44E-05 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
Some toxicological studies have found that TCEP and TEHP have carcinogenic potential and indoor dusts vary according to the indoor environments studied[56-58]. ILCR values were estimated in the three cities and for the different age groups [Table 3, Figure 4]. The ILCRs estimated by MCS were higher via dust ingestion than those via dust dermal absorption. The guideline recommended by US-EPA as safe limit for ILCR is a value of 1 × 10-6[39]. ILCR values estimated for TCEP ranged from 1.14 × 10-5 for infants in Bogotá to 4.27 × 10-4 for adults in Cartagena, while ILCRs for TEHP ranged from 8.79 × 10-7 for infants in Bogotá to 1.03 × 10-5 for adults in Bogotá. Due to the assumed longer lifetime exposure for adults, ILCR values were 40 and 12 times higher in adults than in infants for TCEP and TEHP, respectively. In a worst-case scenario and assuming the highest dust intake and dermal absorption, ILCRs estimated at 95th percentile were 1.39 × 10-3 for TCEP in Cartagena and 1.71 × 10-5 for TEHP in Bogotá. Because mean and 95th percentiles were higher than the safe limit of 1 × 10-6, there is a moderate probability of cancer risk through TCEP and TEHP exposure by dust ingestion and dermal absorption, which is a cause of concern for the public health. A recent study in Saudi Arabia[55] estimated a cancer risk from OPE exposure via dust similar to our estimations for the Colombian population. Our ILCR results were three orders of magnitude higher than those reported for Saudi Arabia.
Figure 4. Cumulative distribution of ILCR simulated by MCS for OPEs (TEHP and TCEP) exposure via home dust: (A) distribution by cities; and (B) distribution by age groups. ILCR: Incremental lifetime cancer risk; MCS: Monte Carlo simulation; OPEs: organophosphate esters; TEHP: tris(2-ethylhexyl) phosphate; TCEP: tri(2-chloroethyl) phosphate.
Incremental lifetime cancer risk simulated by Monte Carlo for TEHP and TCEP exposure via home dust
| OPEs | Age group | Bogotá | Cartagena | Medellín | |||||||||
| Mean | SD | Median | 95th percentile | Mean | SD | Median | 95th percentile | Mean | SD | Median | 95th percentile | ||
| TCEP | Infant | 1.14E-05 | 1.34E-05 | 7.31E-06 | 3.32E-05 | 2.45E-05 | 3.71E-05 | 1.34E-05 | 8.16E-05 | 1.26E-05 | 2.29E-05 | 6.30E-06 | 4.30E-05 |
| Toddler | 6.91E-05 | 8.35E-05 | 4.43E-05 | 2.07E-04 | 1.51E-04 | 2.36E-04 | 8.07E-05 | 4.99E-04 | 7.90E-05 | 1.60E-04 | 3.69E-05 | 2.80E-04 | |
| Children | 9.12E-05 | 1.08E-04 | 5.91E-05 | 2.69E-04 | 1.95E-04 | 3.04E-04 | 1.09E-04 | 6.32E-04 | 1.03E-04 | 1.96E-04 | 5.00E-05 | 3.60E-04 | |
| Teenager | 8.10E-05 | 8.22E-05 | 5.56E-05 | 2.28E-04 | 1.73E-04 | 2.30E-04 | 1.03E-04 | 5.45E-04 | 9.20E-05 | 1.53E-04 | 4.77E-05 | 3.09E-04 | |
| Adult | 1.98E-04 | 2.01E-04 | 1.36E-04 | 5.65E-04 | 4.27E-04 | 5.73E-04 | 2.51E-04 | 1.39E-03 | 2.23E-04 | 3.61E-04 | 1.16E-04 | 7.72E-04 | |
| TEHP | Infant | 8.79E-07 | 6.46E-07 | 6.97E-07 | 2.05E-06 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 |
| Toddler | 5.64E-06 | 4.14E-06 | 4.51E-06 | 1.31E-05 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
| Children | 6.49E-06 | 4.57E-06 | 5.25E-06 | 1.45E-05 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
| Teenager | 4.23E-06 | 1.53E-06 | 3.96E-06 | 7.03E-06 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
| Adult | 1.03E-05 | 3.69E-06 | 9.66E-06 | 1.71E-05 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | 0.00E-00 | |
This study only focused on the OPE exposure through contaminated dust. However, it is important to note that OPE exposure also occurs in other indoor environments (workplaces), as well as by other routes, such as air inhalation and food and beverage ingestion. The sum of all these exposures should increase the estimated risk, both for non-carcinogenic (HQ) and carcinogenic (ILCR) values, which would lead to a higher risk of harmful effects on human health. Different research studies have determined that prolonged human exposure to OPEs can cause adverse effects such as neurotoxicity, reproductive toxicity, carcinogenicity, and endocrine disruption.
It is important to make a special mention of the many uncertainties associated with the estimations of OPE exposure because various factors such as personal habits, occupation, dietary preferences, variation of OPE concentrations from room-to-room, continuous use of consumer products containing OPEs, time spent indoors or outdoors, seasonal dust variation, skin exposure, or ventilation can all affect the magnitude of the exposure.
CONCLUSIONS
The occurrence of OPEs in dust from homes and cars in three Colombian cities was reported for the first time. Sixteen OPEs were detected at concentrations up to 599 μg/g, being the levels similar to those reported in other countries around the world. Levels in car dust samples were higher than those of home dust, but the most contributing OPE compounds were the same in both microenvironments: TCIPP, TDClPP, TPHP, TBOEP, and TCEP.
The EDIs through dust ingestion and dermal absorption were estimated for five different age groups. In general, EDI values decreased with age. Moreover, dust ingestion was the dominant pathway for infants and toddlers, whereas dermal absorption was the dominant pathway for teenagers and adults.
Non-carcinogenic risk was evaluated by the estimation of HQ values using MCS, which provided quantitative description of the human health risk of OPE exposure via dust ingestion and dermal absorption. Obtained mean values ranged from 0 to 0.014, being always below the safe value of 1. Moreover, the carcinogenic risk was also estimated by the ILCRs obtained through MCS, showing higher values for dust ingestion than those for dermal absorption. ILCRs ranged from 1.14 × 10-5 to 8.79 × 10-7, being for many cases higher than the safe limit value (1 × 10-6). Due to the assumed longer lifetime exposure for adults, ILCR values were forty and twelve times higher in adults than infants for TCEP and TEHP, respectively.
Estimated ILCR values for TCEP and TEHP showed that these populations are exposed to moderate cancer risk, being a cause of concern for the public health. In this context, adopting the precautionary principle, it would appear desirable to reduce overall human exposure to OPEs. Environmental legislation should pay more attention to this health problem and act with measures aimed at reducing the degree of human exposure to OPEs.
DECLARATIONS
Authors’ contributions
Made contributions to the conception and design of the study: Eljarrat E, Johnson-Restrepo B
Performed chemical analysis: Olivero-Verbel R
Performed data analysis interpretation and writing of the manuscript: Eljarrat E, Johnson-Restrepo B, Olivero-Verbel R
Provided funding for sampling: Johnson-Restrepo B
Provided funding and material and instrumentation for the experiments: Eljarrat E
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported by the European project INTERWASTE (ID 734522, H2020-MSCA-RISE/0253), the MCIN/AEI/10.13039/501100011033 (Grant CEX2018-000794-S), the Generalitat de Catalunya (Consolidated Research Group 2017 SGR 1404), the Ministerio de Ciencia, Tecnología e Innovación from Colombia (Grant No. 110759634967) and the Universidad de Cartagena.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
Supplementary Materials
REFERENCES
1. de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere 2002;46:583-624.
2. Alaee M. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int 2003;29:683-9.
3. Saito I, Onuki A, Seto H. Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air 2007;17:28-36.
4. Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 2009;76:542-8.
5. Khan MU, Li J, Zhang G, Malik RN. First insight into the levels and distribution of flame retardants in potable water in Pakistan: an underestimated problem with an associated health risk diagnosis. Sci Total Environ 2016;565:346-59.
6. Li HL, Liu LY, Zhang ZF, et al. Semi-volatile organic compounds in infant homes: Levels, influence factors, partitioning, and implications for human exposure. Environ Pollut 2019;251:609-18.
7. Wang R, Tang J, Xie Z, et al. Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, north China. Environ Pollut 2015;198:172-8.
8. Mizouchi S, Ichiba M, Takigami H, et al. Exposure assessment of organophosphorus and organobromine flame retardants via indoor dust from elementary schools and domestic houses. Chemosphere 2015;123:17-25.
9. Meyer J, Bester K. Organophosphate flame retardants and plasticisers in wastewater treatment plants. J Environ Monit 2004;6:599-605.
10. Rodil R, Quintana JB, Concha-Graña E, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012;86:1040-9.
11. Li J, He J, Li Y, et al. Assessing the threats of organophosphate esters (flame retardants and plasticizers) to drinking water safety based on USEPA oral reference dose (RfD) and oral cancer slope factor (SFO). Water Res 2019;154:84-93.
12. Li W, Wang Y, Asimakopoulos AG, et al. Organophosphate esters in indoor dust from 12 countries: concentrations, composition profiles, and human exposure. Environ Int 2019;133:105178.
13. Giulivo M, Capri E, Eljarrat E, Barceló D. Analysis of organophosphorus flame retardants in environmental and biotic matrices using on-line turbulent flow chromatography-liquid chromatography-tandem mass spectrometry. J Chromatogr A 2016;1474:71-8.
14. Garcia-Garin O, Sala B, Aguilar A, et al. Organophosphate contaminants in North Atlantic fin whales. Sci Total Environ 2020;721:137768.
15. Canbaz D, Logiantara A, van Ree R, van Rijt LS. Immunotoxicity of organophosphate flame retardants TPHP and TDCIPP on murine dendritic cells in vitro. Chemosphere 2017;177:56-64.
16. Du Z, Wang G, Gao S, Wang Z. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: by disturbing expression of the transcriptional regulators. Aquat Toxicol 2015;161:25-32.
17. Voorhees JR, Rohlman DS, Lein PJ, Pieper AA. Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds. Front Neurosci 2016;10:590.
18. Hales BF, Robaire B. Effects of brominated and organophosphate ester flame retardants on male reproduction. Andrology 2020;8:915-23.
19. Wang C, Chen H, Li H, Yu J, Wang X, Liu Y. Review of emerging contaminant tris(1,3-dichloro-2-propyl)phosphate: Environmental occurrence, exposure, and risks to organisms and human health. Environ Int 2020;143:105946.
20. WHO (World Health Organization). Tri-n-butyl phosphate, Geneva, Switzerland. Environmental Health Criteria 112. Available from: https://inchem.org/documents/ehc/ehc/ehc112.htm [Last accessed on 2 Mar 2022].
21. Wu M, Yu G, Cao Z, et al. Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere 2016;150:465-71.
22. Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat Toxicol 2013;134-135:104-11.
23. Zhao F, Wang J, Fang Y, et al. Effects of tris(1,3-dichloro-2-propyl)phosphate on pathomorphology and gene/protein expression related to thyroid disruption in rats. Toxicol Res (Camb) 2016;5:921-30.
24. IARC (International Agency for Research on Cancer). IARC monographs on the evaluation of carcinogenic risks to humans. Available from: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono77.pdf [Last accessed on 2 Mar 2022].
25. European Commission. European Union Risk Assessment Report. Tris (2-chloroethyl) phosphate, TCEP. Available from: https://echa.europa.eu/documents/10162/2663989d-1795-44a1-8f50-153a81133258 [Last accessed on 2 Mar 2022].
26. Wei GL, Li DQ, Zhuo MN, et al. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut 2015;196:29-46.
27. Sakhi AK, Cequier E, Becher R, et al. Concentrations of selected chemicals in indoor air from Norwegian homes and schools. Sci Total Environ 2019;674:1-8.
28. Ageel HK, Harrad S, Abdallah MA. Occurrence, human exposure, and risk of microplastics in the indoor environment. Environ Sci Process Impacts 2022;24:17-31.
29. Abdallah MA, Covaci A. Organophosphate flame retardants in indoor dust from Egypt: implications for human exposure. Environ Sci Technol 2014;48:4782-9.
30. Li W, Shi Y, Gao L, Wu C, Liu J, Cai Y. Occurrence, distribution and risk of organophosphate esters in urban road dust in Beijing, China. Environ Pollut 2018;241:566-75.
31. Sun Y, Liu LY, Sverko E, et al. Organophosphate flame retardants in college dormitory dust of northern Chinese cities: occurrence, human exposure and risk assessment. Sci Total Environ 2019;665:731-8.
32. Zhou L, Hiltscher M, Gruber D, Püttmann W. Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: comparison of concentrations and distribution profiles in different microenvironments. Environ Sci Pollut Res Int 2017;24:10992-1005.
33. U.S. Environmental Protection Agency. Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. Available from: https://www.epa.gov/sites/default/files/2013-09/documents/agegroups.pdf [Last accessed on 2 Mar 2022].
34. U.S. Environmental Protection Agency. HPV Chemical Hazard Characterizations. Available from: http://iaspub.epa.gov/oppthpv/hpv_hc_characterization.get_report [Last accessed on 2 Mar 2022].
35. U.S. Environmental Protection Agency. EPA’s Exposure Factors Handbook (EFH). Available from: https://www.epa.gov/expobox/about-exposure-factors-handbook [Last accessed on 2 Mar 2022].
36. Maceira A, Borrull F, Marcé RM. Occurrence of plastic additives in outdoor air particulate matters from two industrial parks of Tarragona, Spain: human inhalation intake risk assessment. J Hazard Mater 2019;373:649-59.
37. Wang Y, Yao Y, Han X, Li W, Zh H, et al. Organophosphate di- and tri-esters in indoor and outdoor dust from China and its implications for human exposure. Sci Total Environ 2019;700:134502.
38. U.S. Environmental Protection Agency. Exposure Factors Handbook 2011 Edition (Final Report). Available from: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 [Last accessed on 2 Mar 2022].
39. U.S. Environmental Protection Agency. Provisional Peer Reviewed Toxicity Values for Tris(2-ethylhexyl)phosphate (CASRN 78-42-2). Available from: https://cfpub.epa.gov/ncea/pprtv/documents/Tris2ethylhexylphosphate.pdf [Last accessed on 2 Mar 2022].
40. U.S. Environmental Protection Agency. Provisional Peer Reviewed Toxicity Values for Triphenylphosphine oxide (CASRN 791-28-6). Available from: https://cfpub.epa.gov/ncea/pprtv/documents/TriphenylphosphineOxide.pdf [Last accessed on 2 Mar 2022].
41. U.S. Environmental Protection Agency. Provisional Peer-Reviewed Toxicity Values for Tris(2-chloroethyl)phosphate (TCEP) (CASRN 115-96-8). Available from: https://cfpub.epa.gov/ncea/pprtv/documents/Tris2chloroethylphosphate.pdf [Last accessed on 2 Mar 2022].
42. U.S. Environmental Protection Agency. Provisional Peer-Reviewed Toxicity Values for Tris(1-chloro-2-propyl)phosphate (CASRN 13674-84-5). Available from: http://www.epa.gov/dfe/pubs/projects/flameret/about.htm [Last accessed on 2 Mar 2022].
43. ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for Phosphate Ester Flame Retardants. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp202.pdf [Last accessed on 2 Mar 2022].
44. US-EPA. Risk assessment guidance for Superfund. Volume I, Human health evaluation manual. Part A. Available from: https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf [Last accessed on 2 Mar 2022].
45. Kim UJ, Wang Y, Li W, Kannan K. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ Int 2019;125:342-9.
46. Esplugas R, Rovira J, Mari M, et al. Emerging and legacy flame retardants in indoor air and dust samples of Tarragona Province (Catalonia, Spain). Sci Total Environ 2022;806:150494.
47. Tokumura M, Hatayama R, Tatsu K, et al. Organophosphate flame retardants in the indoor air and dust in cars in Japan. Environ Monit Assess 2017;189:48.
48. Tao F, Sellström U, de Wit CA. Organohalogenated Flame Retardants and Organophosphate Esters in Office Air and Dust from Sweden. Environ Sci Technol 2019;53:2124-33.
49. Zhou L, Püttmann W. Distributions of organophosphate flame retardants (OPFRs) in three dust size fractions from homes and building material markets. Environ Pollut 2019;245:343-52.
50. Ali N, Dirtu AC, Van den Eede N, et al. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere 2012;88:1276-82.
51. Tajima S, Araki A, Kawai T, et al. Detection and intake assessment of organophosphate flame retardants in house dust in Japanese dwellings. Sci Total Environ 2014;478:190-9.
52. Quednow K, Püttmann W. Temporal concentration changes of DEET, TCEP, terbutryn, and nonylphenols in freshwater streams of Hesse, Germany: possible influence of mandatory regulations and voluntary environmental agreements. Environ Sci Pollut Res Int 2009;16:630-40.
53. Chen Y, Cao Z, Covaci A, Li C, Cui X. Novel and legacy flame retardants in paired human fingernails and indoor dust samples. Environ Int 2019;133:105227.
54. Wang G, Liu Y, Zhao X, Tao W, Wang H. Geographical distributions and human exposure of organophosphate esters in college library dust from Chinese cities. Environ Pollut 2019;255:113332.
55. Kanchanapiya P, Nilyok B, Songngam S, Olapiriyakul S. Organophosphate flame retardants in car dust from Thailand and implications for human exposure. IJCEA 2021;12:1-6.
56. WHO. EHC 218: Flame Retardants: Tris(2-butoxyethyl) phosphate, Tris(2-Ethylhexyl) Phosphate and Tetrakis (Hydroxymethyl) Phosphonium Salts. Available from: http://apps.who.int/iris/bitstream/handle/10665/42248/WHO_EHC_218.pdf?sequence=1 [Last accessed on 2 Mar 2022].
57. WHO. EHC 209: Flame Retardants: Tris-(Chloropropyl)Phosphate and Tris-(2-Chloroethyl)phosphate. Available from: https://www.who.int/ipcs/publications/ehc/who_ehc_209.pdf [Last accessed on 2 Mar 2022].
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.




















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].