Dynamics of estrogenic activity in an urban river receiving wastewater effluents: effect-based measurements with CALUX
Abstract
Estrogenic substances (ES) in an urban river Zenne (BE) dominated by wastewater effluents were assessed over the course of one year. To measure the bioequivalent (BEQ) 17 β-estradiol (E2) concentrations of ES, the biological effect-based methodology - the Chemical-Activated LUciferase gene eXpression (CALUX) bioassay was used. Daily water discharges were collected from January 2015 to February 2016 at or near the sampling stations in the Brussels Capital Region. An annual water budget shows that approximately 50% of the Zenne River flow downstream is from wastewater effluent. The estrogenic activity and yearly average ES load in influents and effluents of wastewater treatment plants (WWTPs) located in the North and South, combined sewer overflows (CSOs) and the Zenne River, were assessed for upstream and downstream of two WWTPs of Brussels. Both WWTPs with activated sludge treatment remove more than 90% of the ES. The influent concentrations of ES at the South and North WWTPs ranged from 30-359 and 18-55 ng E2 eq./L, respectively. The effluent concentrations of ES ranged from 1.0-2.1 and 1.1-6.6 ng E2 eq./L at WWTP-S and -N, respectively. The yearly average ES loads were 0.05-0.14 and 0.39-1.5 g E2 eq./d for WWTP-S and -N, respectively. The temporal variation of E2-eq concentrations at the river stations Z3 and Z5 (upstream) ranged from 1 to 2 ng E2 eq./L, while the ES activity at sites Z9 and Z11 (downstream) varied from 2-17 ng E2 eq./L and from 1-8 ng/L ng E2 eq./L, respectively. The relative ES loads to the Zenne River are as follows: WWTPs (31%), CSOs (27%), upstream Zenne (15%), a missing source (14%), and local tributaries (13%). ES in the Zenne River behave in a pseudo-persistent manner because of continuous input from the WWTPs and slow degradation in the 18 km river stretch. The BEQ concentration of E2 exceeds the EU environmental quality standards (EQS) of 0.4 ng E2/L throughout the Zenne River.
Keywords
INTRODUCTION
Organic chemical contamination of freshwaters, a major societal and ecosystem health concern, derives from domestic and industrial effluents, agriculture runoff, energy and transport sectors, and atmospheric deposition[1,2]. Since the 1950s and 1970s, many challenging chemicals have entered the environment, often referred to as ‘legacy’ contaminants, such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), chlorinated dioxins and furans, and chlorinated pesticides. Many of these substances have been banned, restricted, or planned for phase-out by national and/or international conventions. This also applies to the persistent, toxic, and bio-accumulative perfluorinated compounds - PFAS - which are widely distributed in the environment[3,4]. However, detectable concentrations of contaminants can still be found in global surface waters, sediments, and aquatic organisms in lakes, rivers, and oceans[5-9]. Currently, there is growing attention on ‘‘chemicals of emerging concern” (CECs), which are previously unknown contaminants that possess significant and continuous input rates, are persistent in the environment, and are frequently classified as Persistent, Bio-accumulative and Toxic (PBTs) and endocrine disruptors[9-14]. Specific examples include synthetic and natural hormones (estrogens), anti-inflammatory drugs, antioxidants, antibiotics, pesticides, fungicides, and pre- and post-emergent herbicides. These chemicals tend to be more polar and water-soluble than traditional compounds and are inherently biologically active and transformed without necessarily losing potency. Surveys of CECs in European waters focusing on polar compounds have been reported recently[15-17]. Current data show that European wastewater effluents and receiving river waters contain a wide variety of pharmaceuticals and both naturally occurring and synthetic estrogens[10,18-29]. In the European Union, the Water Framework Directive (WFD) establishes environmental quality standards (EQS) that protect the most sensitive aquatic organisms through rigorous risk assessments, leading to the creation of Priority Substances (PS) and Priority Hazardous Substances (PHS) lists. Assessment of chemical water status is presently compound-specific, while chemical mixtures may produce combined toxic effects even though individual substances are not exceeded[1,30]. In 2013, an amendment to the WFD established a mechanism – a Watch List - which aimed to provide targeted, high-quality, EU-wide concentrations of substances of possible concern and not yet listed as PHS[31]. The Watch List contains CECs for which the available monitoring data are insufficient or of insufficient quality to conduct an appropriate risk assessment. In 2015, two estrogens were added to this list, 17α-ethynyl estradiol (EE2) and 17β-estradiol (E2), which introduced a new dimension in EU aquatic assessments. While the proposed EQS for EE2 and E2 are 0.035 and 0.4 ng/L, respectively, these hormones are extremely difficult to detect analytically in surface waters and effluents. In practice, they are often not quantifiable.
Ongoing monitoring efforts of EU member states now include estrone (E1), with a target of 3.6 ng/L. EDCs, particularly estrogens, adversely affect the reproduction and development of aquatic biota[31-35]. Wastewater treatment plant (WWTP) effluents are important sources of estrogenic endocrine disruptor chemicals in receiving river waters[25,26,28,36,37]. Although WWTPs are frequently highly effective in reducing incoming loads of these estrogens, continuous loading amounts and patterns can result in concentrations of over 1-50 ng/L E2[25,37]. Measurements with effect-based methods (EBMs) show estrogenic activity at concentrations in surface waters of 0.5 to over 5 ng/L of bioequivalent E2[21,28,38]. Using a geographic-based model linked to E2 and EE2 usage and emissions in the EU, significant reaches of EU rivers may exceed the proposed WFD EQS for EE2 of 0.035 ng/L[39]. An alternative and/or complementary monitoring strategy involving EBMs to assess the chemical quality and status of surface waters has been suggested[19-21,40] and is being evaluated in tandem with compound-specific analyses[29]. The Common Implementation Strategy of the WFD has recognized the advantage of EBMs in the monitoring of chemical pollutants and has recommended their use with compound-specific measurements[41-45].
The development and evaluation of effects-based surveillance tools (such as in vitro testing) can support the establishment of chemical analysis and surveillance strategies. These tools offer several advantages, including the reduction in monitoring and analysis costs and the ability to prioritize targeted chemical analysis based on effects assessment, which directly address the issue of the impact of chemicals on aquatic organisms. Over the past decade, the need for effects-based monitoring tools to measure estrogenic activity in surface waters has gained greater importance[20,41,43,46-47].Recently, Konemann et al. described the relationships between effects-based and chemical analysis methods for monitoring E1, E2, and EE2 in an EU-wide study[28]. The most important finding is that the bioequivalent (BEQ) E2 concentrations provided by EBMs, including the Chemical-Activated LUciferase gene eXpression (CALUX) bioassay and chemical analytical concentrations of E2 and EE2, were strongly correlated and in nearly perfect agreement, supporting the recommendation to integrate EBMs into EU-WFD water monitoring programs.
The overall objective of this study was to describe the BEQ E2 activities, loadings, and dynamics of estrogenic substances (ES) in an urban European river dominated by wastewater effluents. The study was conducted in the Zenne River, which flows through Brussels (BE) and is anchored on the south and north by two major WWTPs. There are few studies that report on temporal inflows and discharges of estrogenic chemicals from WWTPs and link them to temporal and spatial concentrations in receiving waters. The specific objectives of this study were to assess, over a one-year period, (i) the estrogenic activity in both WWTP influents and effluents; (ii) the activity and fluxes to the Zenne River from the WWTP effluents and their seasonal variability within the city of Brussels; and (iii) the contribution of wastewater effluent discharges versus other sources, including the main river, tributaries, and combined sewer overflows (CSOs).
MATERIALS AND METHODS
Study Area
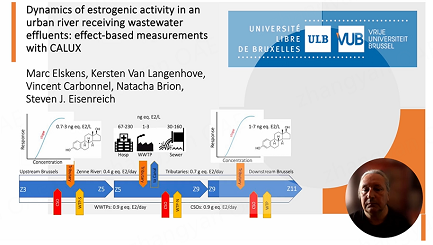
The Zenne River is a small, temperate, rain-fed, and lowland river that belongs to the Scheldt River basin, draining an area of approximately 1160 km2. Typical land use in the upstream basin is dominated by arable land (51%) and pastures (18%), while the central and downstream basin is mostly urban (19%) and contains the Brussels conurbation (over 1.2 million inhabitants) and some forests (10%)[48]. The river basin is densely populated (1480 inh/km²), and its hydro-morphology has been significantly altered by human activities. Important modifications include the vaulting of a 7 km stretch in the center of Brussels, the diversion of a part of the stream flow to feed the Charleroi-Brussels Canal, and the use of the Canal as a bypass channel during extreme flow events[48]. The main river stretch extends over 60 km from the confluence of the Zenne-Sennette rivers in Tubize, parallel to the Canal, through the Brussels Capital Region from South to North and downstream to Zennegat at the confluence with the Dijle River. Upstream from Brussels, the Zenne has an average annual discharge of 3.7 m3/s, with 10 m3/s at the outlet of the basin, showing a dominant contribution of urban water flows (on average, 50% of the water downstream Brussels are urban WWTP waters). The study area shown in Figure 1 is a 28.3 km stretch from the main river flowing from Z3-Beersel (km 0 -reference station for distances) to Z11-Eppegem (km 28.3) and crossing the Brussels Capital Region over a large part of the distance (km 6 to km 20.6). Several small tributaries enter the Zenne study area - the Zuunbeek (km 5.8), the Woluwe (km 21.8), and the Tangebeek (km 27.8) - while water exchange with the Canal can occur through three overflow structures: the Aa overflow (from Zenne to Canal and from Canal to Zenne, km 6.9), the Porte de Ninove overflow (from Zenne to Canal, km 12.2) and the Vilvoorde overflow (from Canal to Zenne, km 26.4). Within the study area, there are four WWTPs, three of which discharge into the Zenne mainstream and one into the Tangebeek, close to the confluence with the Zenne River [Figure 1]. From upstream to downstream: WWTP Beersel (45,000 eq. inhab., since 1990, to Zenne), Brussels-South (360,000 eq. inhab., since 2000, to Zenne), Brussels-North (1,100,000 eq. inhab., since 2007, to Zenne), and Grimbergen (90,000 eq. inhab., since 2007, to Tangebeek). All sewer systems connected to the WWTP are combined sewer systems (i.e., they collect domestic sewage and surface rainwater) and are equipped with overflow structures (CSO) that are connected to the Zenne River main stem and some tributaries. The most important CSO in terms of volumes and frequency is the Lion-CSO, located in the Northern part of Brussels. The two major WWTPs are both located in Brussels – one at its southern border (Brussels South - WWTP-S) and the other at its northern border (Brussels North - WWTP-N). The urban wastewater collection system in Brussels consists of two drainage basins, with approximately 25% of the area serviced by the South Basin and the rest serviced by the North Basin. At the time of the study, WWTP Brussels-South functioned as a secondary treatment facility without the removal of N and P, while WWTP Brussels-North was a tertiary treatment facility. Both stations have a biological treatment line for dry weather flows and a rain treatment line for excessive wet weather flows.
Sample Collection
Twelve sampling campaigns were conducted between January 2015 and February 2016 to assess the estrogenic activity and loads in the Zenne River. 24-hour composite samples were collected from the WWTP-S and WWTP-N influent and effluent and from hospital effluent (UZ Brussels hospital). River water samples were taken from the Zenne River at the monitoring stations Z3, Z5, Z9, and Z11 [Figure 1]. Sampling stations Z3 and Z11 are located just outside of Brussels and were selected as the most upstream and downstream boundaries of the study area, respectively. Sampling stations Z5 and Z9 captured the influence of the effluent from the WWTP-S and WWTP-N, respectively. This enabled the study of the impact of each WWTP on the estrogenic activity measured in the Zenne River. All river stations were sampled for physicochemical parameters (temperature, conductivity, dissolved oxygen, suspended particulate matter, and pH) and estrogenic activity. Daily water discharges were collected for the whole observation period at or nearby the sampling stations [see hydrometric data in Supplementary Materials]. Surface water samples were collected with a sampling bucket in the middle of the stream. Water quality parameters were measured in situ with a VWR MD 8,000 L Digital Multi-Parameter Instrument, including pH, conductivity, dissolved oxygen, and temperature. Water samples were transferred to 5L sampling containers (HDPE) and preserved at ~4 oC during transport to the laboratory. The hydrographs of the discharge measured at each station indicate that most sampling occurred at flows equal to (Nov15 to Feb16) or lower than (Apr-May16 and Jul-Sep15) the mean flow [Figure 2]. Only flows in January and Jun15 were significantly higher than the mean flow rate (~2x the mean flow).
Determination of suspended particulate matter concentration
Two-liter water samples were filtered through a standard glass-fiber paper filter according to the procedure described in the study by Pfannkuche and Schmidt[49]. Filters were dried in an oven at 105 °C overnight and left to cool to room temperature in a desiccator. Then, the filters were returned to room temperature (20 °C, RH 50%), and the weight gain was determined by gravimetry.
Assessment of estrogenic activity using ERα-CALUX.
Water samples for measurement of estrogenic activity were filtered through pre-weighed glass fiber filters (GFF, 0.7 μm, Whatman) to remove suspended matter. The resulting filtrates were then passed through solid-phase extraction (SPE) columns (HLB Oasis 6 cc glass cartridge, Waters) to isolate and concentrate estrogenic chemicals of interest. The sample volumes ranged from 50 mL (hospital) and 150 mL (WWTP influent) to 800 mL (Zenne River and effluents of the WWTPs) and were processed as described by Vandermarken et al. using methanol/methyl-ter-butyl ether (10/90) as the elution solvent[38]. The recombinant human breast cancer cell line VM7Luc4E2 (variant MCF7, formerly known as BG1Luc4E2) was used to determine estrogenic activities. These cell lines express ERα endogenously but lack functional ERβ[50-51]. The bioanalytical procedure is briefly described in the Supporting Information section.
Data analysis and bioequivalent E2 concentration
A four-parameter logistic function was fitted to the data points (RLUs of the standard solutions or dilutions of the samples as a function of the concentrations in amount per well) using a weighted least squares regression[52]:
where a, b, c, and d are the parameters of the model, and where the concentration x corresponds to the explanatory variable and RLU to the response variable; a and d respectively represent the lower and upper asymptote, b the slope or Hill coefficient, and c is the half-effective concentration or EC50.
The estrogenic activity of each sample was converted into a bioequivalent (BEQ) concentration (ng E2 eq./L) and calculated according to (2):
where subscript std and spl represent standard and sample, respectively, and EC50 is the half-effective concentration.
Quality control and quality assurance
Quality control (QC) samples were systematically performed in triplicate on the 96-well plates. They consist of the standard at the half-maximal effective concentration (EC50). The recovery rates for these quality checks ranged between 89%-120%, which were considered satisfactory. Furthermore, control charts were used to monitor the characteristic parameters of dose-response curves for E2 (n = 10), such as EC50 and Hill slope. The precision of these parameters under repeatability conditions (rsd%) were 4 and 11%, respectively. Finally, the recovery rate for a spiked MQ water sample with E2 at 16 ng/L (n = 6) was 87% ± 2.3 % with a repeatability of less than 5%. For samples with activity below the threshold values of 10 and 20%, they are designated as < LoD and < LoQ, respectively. These values have been calculated using the following formulas: (i) LoD = Blank + 3 standard error of blank; (ii) LoQ = Blank + 10 standard error of blank. Here, the blank represents the value of the medium + DMSO (1%).
Hydrometric data
Hydrometric data were collected from January 1, 2015, to August 2, 2016. Continuous water height, velocity, and flow measurements were summarized in Supplementary Materials. The annual pollutant load for ES was calculated according to Elwan et al.[53]:
where Q = annual discharge, Qi = discharge on sampling day i, BEQi = BEQ on sampling day i.
Statistical data treatment
All statistical analyses were performed using the XLSTAT software (Addinsoft). Principal component analysis (PCA) was conducted to assess the spatial distribution of estrogenic activity in the water samples. The extraction procedure was based on a normalized PCA using the correlation matrix with Varimax rotation and Kaiser normalization. All the statistical tests were performed at a significance level of 5%.
RESULTS
Water flow and budget
Daily Zenne River discharges on the sampling days are presented in Figure 2A. Observed variations show a typical pattern of higher discharge during the winter months (December-February) and lower during the spring-summer months (April-October), except for the sampling campaigns of June (all stations) and August (only downstream Brussels) 2015, which experienced heavy rainfall events. During these summer rainfall events, important CSO discharges were observed at Lion and SPLeeuw [Figure 2D]. Additionally, the discharge of the WWTPs follows the same seasonal trend as the river discharge [Figure 2C]. Finally, the tributaries follow different seasonal trends: while the Zuunbeek discharge behaves similarly to the Zenne River, the Tangebeek and Woluwe discharges remain relatively constant over the seasons, suggesting that they are probably dominated by groundwater flow rather than rainfall [Figure 2B]. The consistency of water discharge data was checked for the Zenne River between Z3 and Z5, Z5 and Z9, and Z9 and Z11 by constructing hydrological budgets, as described in Supplementary Materials. The relative importance of river, tributaries, WWTPs, and CSOs water flows is best evaluated by considering all daily discharge data for the whole year of 2015 [Figure 3]. Between Z3 and Z5, the most important contributors to the river flow are the Zuunbeek (11%) and the outflow of the WWTP Brussels-South (15%). In the central zone between Z5 and Z9, an important part of the flow is diverted to the Canal (17%), and there was significant input from the WWTP Brussels-North (47%). Finally, in the Northern downstream part between Z9 and Z11, there are minor contributions from Woluwe (3%), WWTP Grimbergen (4%), and the Canal waters coming from the siphons of Vilvoorde (5%). We concluded that CSOs contribute only marginally to the overall water budget (≤ 2%). CSOs are typically short-term events linked to heavy local rainfall. They may be insignificant for the water budget on a yearly scale; however, they can be more important on a daily scale. For example, the CSO event in June 2015 represents a contribution of almost 10% of all daily water inputs in Brussels (between Z5 and Z9).
Figure 3. Framework for estimating the water budget in the Zenne River in 2015. The gray arrow represents the differences between the outflow and sum of inflows for each box and for the whole river stretch [Table 1]. Dam3 = 1000 m3
Estrogenic activity and annual average loads in influents and effluents of WWTPs, Hospitals, and CSOs
WWTPs are major sources of estrogenic substances (natural and synthetic) to the receiving waters[13,54-55]. In the Zenne River system near Brussels, the influent and effluent samples of the WWTP-S and -N were collected. The influent concentrations of ES at the South and North WWTPs ranged from 30-359 and 18-55 ng E2 eq./L, respectively [Figure 4]. The effluent ES concentrations ranged from 1.0-2.1 and 1.1-6.6 ng E2 eq./L at WWTP-N and -S, reflecting annual removal efficiency rates between 86 and 99%, respectively. The yearly average ES loads were estimated from the measured equivalent concentrations and the volumetric flows [Equation 3]. The range in mean flows at WWTP-S was 51-141 103 m3/d, 5-7 times less than the flow at WWTP-N, which ranged from 223-654 103 m3/d. Accordingly, the effluent ES loads discharged to the Zenne River were 0.05-0.14 and 0.39-1.5 g E2 eq./d for WWTP-S and -N, respectively [Table 1].
Figure 4. Bioequivalent E2 concentration on the sampling days and at sampling locations in WWTP-S, WWTP-N, and UZ Brussels hospital. WWTP: wastewater treatment plant.
Input and output data for water and BEQ E2 [Equation 2] budgets in 2015 for the two wastewater treatment plants (influent and effluent), Zenne River sampling sites, tributary sites, and CSOs
| Annual average dam3/day(4) | min -max dam3/day | Annual average g/day | min - max g/day | |
| WWTP-S (n = 12) Influent Effluent RE(1) | 65 | 51-142 | 5.4 0.09 98% | 2.1-24 0.05-0.14 96%-99% |
| WWTP-N (n = 12) Influent Effluent RE(1) | 299 | 223-655 | 11 0.71 94% | 7.3-23 0.39-1.5 86%-96% |
| Hospital(2) (n = 9) Effluent(3) | 10-3 per bed | 10-3 per bed | 0.09 | 0.06-0.19 |
| CSOs (n = 12) SP Leeuw Lion Grimbergen | 5 15 2 | 0-225 0-122 0-3 | 0.16 0.34 0.26 | 0-0.24 0-2.16 0-0.36 |
| Tributary (n = 12) Zuunbeek Tangebeek Woluwe | 47 5 22 | 3-151 2-12 12-26 | 0.06 0.01 0.03 | 0-0.18 0-0.02 0.01-0.07 |
| Canal (n = 12) Aa (in) Aa (out) Vilvoorde (in) | 10 116 40 | 0-28 3-491 0-120 | 0.02 0.13 0.37 | 0-0.08 0-0.49 0-2.0 |
| Zenne (n = 12) Z3 Z5 Z9 Z11 | 320 426 677 782 | 114-730 105-1052 201-1583 359-1323 | 0.42 0.55 3.4 2.8 | 0.12-1.1 0.14-1.1 0.29-26 0.66-9.2 |
The median loads, normalized to the number of equivalent inhabitants in the sub-sewerage systems, are approximately 0.65 µg eq. E2/d/1000 eq. inhabitants at WWTP-N compared to approximately 0.25 µg eq. E2/d/1000 eq. inhabitants at WWTP-S. This suggests that there are additional sources of estrogenic activity in the WWTP-N collection system. Effluents generated by hospitals could be one of these sources, given the nature and importance of ES they may contain and the volumes of effluents produced (of the order of 1 m3/day/active bed). For example, the ES concentrations in raw effluents from UZ Brussels hospital ranged from 67-231 ng E2 eq./L [Figure 4] and were, on average, more than twice the activity measured in the influents of the WWTPs. Hospital effluents are diluted and transported through conventional sewage networks. Assuming a removal efficiency of approximately 90% at the domestic wastewater treatment facilities and roughly 8,500 active hospital beds in the Brussels Capital Region, the yearly average ES loads related to the hospital effluents would contribute to about 0.09 g E2 eq./day [Table 1]. Other possible sources of estrogenic activity in the Zenne River are CSOs, which have yearly average values ranging from 0.16 to 0.34 g E2 eq./day [Table 1].
Estrogenic activity and loads in the surface water of the Zenne.
The time-dependent changes in the BEQ E2 concentration at the four sampling stations in the Zenne River are shown in Figure 5. The concentration levels between sites Z3 and Z5 varied from 1 to 2 ng E2 eq./L throughout the year, while the activity varied more significantly at sites Z9 and Z11 with values ranging from 2 to 17 ng E2 eq./L and from 1 to 8 ng/L ng E2 eq./L, respectively.
Figure 5. Bioequivalent E2 concentration (ng/L) at the four sampling locations in the Zenne River - Z3, Z5, Z9, and Z11.
Peaks of ES activity were observed at Z9 and Z11 during the sampling campaigns of June, which experienced heavy rainfall over Brussels and at all stations in August 2015 [Figure 2]. It is noteworthy that the CALUX-measured BEQ E2 concentration in surface water of the Zenne consistently exceeded the proposed E2-EQS of 0.4 ng/L (2018/840/EU). A multivariate analysis, including the water quality parameters (pH, conductivity, dissolved oxygen, temperature, and concentration of suspended matter) and the BEQ E2 concentrations, was carried out, and the results are summarized in Supplementary Materials. The PCA analysis shows a fairly marked seasonal and longitudinal pattern, but there is a positive correlation between BEQ E2 concentration and conductivity (Pearson correlation = 0.76, p < 0.01) throughout the studied river stretch, suggesting that ES behave conservatively, in agreement with Vandermarken et al.[38]. From the upstream (Z3) to the downstream (Z11), the average annual concentration load was lowest, 0.42 g E2 eq./d, at the most upstream station Z3; it increases to 0.55 g E2 eq./d (+ 20%) after receiving effluents from WWTP-S and the Zuunbeek; an additional strong increase (7-fold) to 3.7 g E2 eq./d occurred after receiving effluents from WWTP-N and CSOs; and the activity finally decreases by 24% to 2.8 g E2 eq./d in the most downstream river stretch, which might indicate the presence of removal (self-purification) and/or and sorption processes [Table 1].
DISCUSSION
Main contributors to the ES loads in the Brussels Capital Region
The study area of the Zenne River in the vicinity of Brussels was divided into three boxes for which main inputs and outputs were considered [Figure 3]. The main characteristics of these inputs and outputs are summarized in Table 1, and the following assumptions were made: (i) the CSO waters upstream of WWTP-S have the same BEQ E2 concentrations as in the WWTP-S influents; (ii) the CSO waters in Brussels and downstream Brussels have the same BEQ E2 concentrations as in the WWTP-N influents; (iii) the tributaries and Canal have the same BEQ E2 concentrations as measured in Z3; and (iv) the water flowing to the Canal in Aa has the same BEQ E2 concentrations as in Z5. The first two assumptions are related to the definition of CSOs, which are systems designed to occasionally overflow and discharge excess wastewater directly into neighboring waterways. Therefore, these CSOs contain not only stormwater but also untreated human and industrial waste, urban runoff, and debris. The last two hypotheses are supported by the correlation observed between conductivity and the BEQ concentration in the study area [Figure 5]. The conductivities of the tributaries and Canal are closest to the range observed at Z3, while those of Canal at Aa and Z5 are similar. The other downstream stations, Z9 and Z11, display a higher conductivity. The daily ES loads of the sum of all inputs within the boundaries Z3-Z11 have a yearly average value of 2.8 g E2 eq./d, the percentage distribution of which is shown in Figure 6.
Figure 6. Estrogenic Substance (ES) loads for the Zenne River in the Brussels Capital Region. Top panel: Load calculation with missing water sources. Bottom panel: Refined load calculation after assessment of missing water sources (see text). CSO: combined sewer overflow; WWTP: wastewater treatment plant.
The main contributors to the overall pollution are the WWTPs (31%) and CSOs (27%), followed by the upstream Zenne (15%), a missing source (14%), and the tributaries (13%). The missing source represents possible exchanges between surface and groundwaters[50], direct discharges from unknown sources, unquantified Brussels CSOs for which there are no direct available observations, and the sum of uncertainties related to our assumptions that affect the overall flux estimates.
To refine the annual budget and determine the contributions of these unknown sources, the BEQ concentrations in the “missing water sources” were approximated by dividing the “missing ES load” by the “missing water discharge” in the river stretch between (i) Z5 and Z9, which shows the greatest percentage of water imbalance between outflow and inflow [Supplementary Table 1] and (ii) Z3 and Z11 [Figure 3]. The first estimate provides a value of 51 ng E2 eq./L, which is close to the median value of 48 ng E2 eq./L measured in untreated domestic wastewater. This suggests that between Z5 and Z9, the “missing ES load” is mainly due to unaccounted CSOs. Repeating this calculation for the whole river stretch between Z3-Z11, a concentration of 12 ng E2 eq./L is obtained, which is significantly higher than the concentration measured in the river (approximately 1 ng E2 eq./L) and in treated wastewater (approximately 2 ng E2 eq./L) but lower than in the CSOs. As the concentrations in the river and the treated wastewater are quite similar, the proportions of CSO (x) and “other water sources” (y) can then be estimated from a two-end mixing equation where (51 * x + 1.5 * y) = 12 and x + y = 1, giving the following estimates for CSO (21%) and unknown treated water sources (79%). These data do not fundamentally amend the earlier budget but confirm that the major contributors to ES loads in the Zenne River in Brussels are WWTP-effluents and CSOs [Figure 6]. However, CSOs are short-lived events (from a few minutes to a few hours) and can occasionally be more important. For example, the calculations performed during a heavy rain event in Jun 2015 indicate that CSOs were responsible for approximately 88% of the total ES load to the Zenne for a short period of fewer than 24 hours. Data obtained from Flowbru (http://www.flowbru.be/fr) on the Lion overflow site in 2015 reported that there had been about 120 CSO events per year, characterized by flows ranging from 5.3 to 26 m3/s, with durations ranging from 4 h to 16 h and total water volumes per event between 38,600 to 345,000 m3. Therefore, the impact of CSOs on the ES burden is extremely variable annually and almost unpredictable. While CSOs are generated by flooding, which introduces untreated water into the system, they also dramatically increase the concentration of particles in the water column through erosion and resuspension. In the study area, ES are more abundant in the sediments than in water when considering the weight/weight ratio of the measured concentration, namely, BEQ-E2 sorbed (pg/kg)/BEQ-E2 dissolved (pg/L). For the Zenne River, these ES ratios were around 200 to 1,300 L/kg[38]. In dry weather, the concentrations of suspended matter (SPM) is between 50 and 100 mg/L but increase strongly during heavy rainfall up to 600 to 2,000 mg/L[48]. As a result, the BEQ E2 concentration in the water column (dissolved + particles) can be 2-3x higher.
ES removal at WWTPs and in the Zenne river
Quantified ES activity shows that the municipal WWTP-S and -N with an activated sludge system removes more than 90% of ES, similar to other urban treatment facilities[55-58]. The main mechanism for such removal involves biotransformation/biodegradation[38] and sorption processes[58-60]. The fate and transport of ES in the river appear different under dry vs. wet conditions. Vandermarken et al. report that the estimated ES removal half-life in the Zenne is approximately 15 days, too long to significantly impact the measured BEQ E2 concentration in the dissolved phase as the water residence time in the river stretch between Z3 and Z11 is only a few hours[38]. Thus, the dissolved phase ES acts pseudo-conservatively, unlike the particulate phase ES, since the residence time of suspended solids between Z3 and Z11 is largely impacted by sedimentation and resuspension processes[48]. The dynamics and behavior of ES in the Zenne appear, therefore, regulated by the flow conditions: in the event of a strong flood, the sediment acts as a source of ES to the water column, while in dry periods, it acts like a sink.
To summarize, estrogenic activity in the Zenne River (BE), estimated as BEQ E2 concentration using ERα-CALUX, allows us to draw conclusions likely valid for other highly urbanized rivers. In these river systems, the average annual river flow and water quality are dominated by the cleansing of urban wastewater effluents and the removal efficiency at the treatment plants. The presence of Brussels city (approximately 1.2 × 106 inhabitants) on the river course drastically affects the sources of ES via WWTPs and CSOs. Indirect discharges via hospital effluents, despite a high concentration of BEQ-E2, do not seem to play a major role. Under low water discharge (less than 600×103 m/day), the ES loads are quite reduced (0.12 to 0.66 g E2 eq./d). There is more retention, more sedimentation, and a limited amount of urban surface runoff. Under high water discharge (more than 1300×103 m/day), when the importance of CSO increases due to heavy rainfalls, the ES loads increase significantly (1 to 26 g E2 eq./d). There is less retention and an increase of erosion and urban surface runoff. The continuous input of estrogens by the two WWTPs, the relatively short water residence time of this 18 km stretch of the Zenne River, and the experimentally determined low rate of transformation/loss show that the estrogenic activity exhibits conservative-like behavior. The BEQ E2 concentration exceeds the EQS of 0.4 ng E2/L in all reaches of the river. This raises the question of how to interpret the BEQ assessed by CALUX. Although the ERα-CALUX assay does not identify which substances are present in the tested sample, several studies have shown that CALUX results for ES in rivers closely reflect the analytically determined concentrations of E1, E2, and EE2[28,61]. Current monitoring approaches emphasize either targeted exposure or effect detection[62-63]. The CALUX method and bio-active assessment for ES for water quality monitoring contribute to the balance between exposure and effects-based assessments.
The authors would like to express our gratitude to a Referee for bringing to our attention complementary studies on estrogenic activity and concentrations of E2 and EE2 in WWTP effluents and impact on receiving waters: Vajda et al. (2008)[64], Barber et al. (2019)[65], Harraka et al. (2021)[66], and a recent article on EBMs by Neale et al. (2023)[67].
DECLARATIONS
AcknowledgmentsWe would like to express our gratitude to the late Professor Michael Denison, who sadly passed away on 22 March 2022 at the age of 67 after a long illness. Mike was internationally recognized as the ‘father’ of the acclaimed CALUX assay used in the research described in this paper. As a long-time member of the University of California Davis’ Dept of Environmental Toxicology, he spent 20 years educating young toxicologists and perfecting the CALUX bio-activity assay (adopted by the OECD and the US EPA) to detect PCBs, Dioxins, PAHs, estrogens, and more. Mike’s work will live on in his students and colleagues. And he will be missed. The authors would like to acknowledge E. Swartz for her contribution to the fieldwork.
Authors contributionsMade substantial contributions to the design of the work, analysis of data, and co-writing and editing of the manuscript: Elskens M
Made substantial contributions to conducting the fieldwork and analyzing samples: Van Langenhove K
Contributed to the construction of the water budget in the study area: Carbonnel V
Provided key input on the water budget in the Zenne River and analyzed data: Brion N
Contributed to the analysis of data collected and writing and editing of the manuscript: Eisenreich S
Availability of DataResearch data are reported in Supporting Material and available from the First Author.
Financial SupportThis work was funded by the VUB Strategic Research Program and was supported by the Research Foundation - Flanders grant to ME (FWOKN299) and by the Prospective Research for Brussels - INNOVIRIS (Grant BHG/2022-PRB-6)
Conflicts of InterestAll authors declare that they have no conflict of interest.
Ethical approval and consent to participateER-CALUX uses genetically modified eukaryotic cell lines. Elskens M. has obtained ethical approval with biosafety permit nr LABO-417843, Risk Level 1, issued on 29/04/2020 and expiring on 29/04/2030 from Brussels Environment in accordance with the decree of the Government of the Brussels-Capital Region dated 8 November 2001.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Schwarzenbach RP, Escher BI, Fenner K, et al. The challenge of micropollutants in aquatic systems. Science 2006;313:1072-7.
2. Dachs J, Méjanelle L. Organic pollutants in coastal waters, sediments, and biota: a relevant driver for ecosystems during the anthropocene? Estuaries Coasts 2010;33:1-14.
3. ATSDR. Per- and polyfluoroalkyl substances (PFAS) and your health. 2023. Available from: https://www.atsdr.cdc.gov/pfas/health-effects/index.html. [Last accessed on 16 May 2023].
4. Cousins IT, Johansson JH, Salter ME, Sha B, Scheringer M. Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS). Environ Sci Technol 2022;56:11172-9.
5. Eisenreich SJ, Capel PD, Robbins JA, Bourbonniere R. Accumulation and diagenesis of chlorinated hydrocarbons in lacustrine sediments. Environ Sci Technol 1989;23:1116-26.
6. Stockhom convention of persistent organic pollutants 2011. UNEP/POPS/COP.5/INF/27. Available from: http://www.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx. [Last accessed on 16 May 2023].
7. Ankley GT, Coady KK, Gross M, et al. A critical review of the environmental occurrence and potential effects in aquatic vertebrates of the potent androgen receptor agonist 17β-trenbolone. Environ Toxicol Chem 2018;37:2064-78.
8. Masset T, Cottin N, Piot C, Fanget P, Naffrechoux E. PCB mass budget in a perialpine lake undergoing natural decontamination in a context of global change. Sci Total Environ 2019;693:133590.
9. Mudgal S, De Toni A, Lockwood S, Sales K, Backhaus T, Sorenson BH. Study on the environmental risks of medicinal products. Final report, 308 p. Available from: https://health.ec.europa.eu/system/files/2016-11/study_environment_0.pdf. [Last accessed on 16 May 2023].
10. Ankley GT, Brooks BW, Huggett DB, Sumpter JP. Repeating history: pharmaceuticals in the environment. Environ Sci Technol 2007;41:8211-7.
11. Jobling S, Burn RW, Thorpe K, Williams R, Tyler C. Statistical modeling suggests that antiandrogens in effluents from wastewater treatment works contribute to widespread sexual disruption in fish living in English rivers. Environ Health Perspect 2009;117:797-802.
12. Novak PJ, Arnold WA, Blazer VS, et al. On the need for a National (U.S.) research program to elucidate the potential risks to human health and the environment posed by contaminants of emerging concern. Environ Sci Technol 2011;45:3829-30.
13. Richardson SD, Ternes TA. Water analysis: emerging contaminants and current issues. Anal Chem 2014;86:2813-48.
14. Patel N, Khan MDZ, Shahane S, et al. Emerging pollutants in aquatic environment: source, effect, and challenges in biomonitoring and bioremediation - a review. Pollution :2020 6, 99 - 113.
15. Loos R, Gawlik BM, Locoro G, Rimaviciute E, Contini S, Bidoglio G. EU-wide survey of polar organic persistent pollutants in European river waters. Environ Pollut 2009;157:561-8.
16. Silva BF, Jelic A, López-Serna R, Mozeto AA, Petrovic M, Barceló D. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 2011;85:1331-9.
17. Hartmann J, van Driezum I, Ohana D, et al. The effective design of sampling campaigns for emerging chemical and microbial contaminants in drinking water and its resources based on literature mining. Sci Total Environ 2020;742:140546.
19. Tang JY, McCarty S, Glenn E, Neale PA, Warne MS, Escher BI. Mixture effects of organic micropollutants present in water: towards the development of effect-based water quality trigger values for baseline toxicity. Water Res 2013;47:3300-14.
20. Kunz PY, Kienle C, Carere M, Homazava N, Kase R.
21. Kunz PY, Simon E, Creusot N, et al. Effect-based tools for monitoring estrogenic mixtures: Evaluation of five
22. Loos R, Carvalho R, António DC, et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res 2013;47:6475-87.
23. Sumpter JP, Jobling S. The occurrence, causes, and consequences of estrogens in the aquatic environment. Environ Toxicol Chem 2013;32:249-51.
24. Sumpter JP, Johnson AC, Williams RJ, Kortenkamp A, Scholze M. Modeling effects of mixtures of endocrine disrupting chemicals at the river catchment scale. Environ Sci Technol 2006;40:5478-89.
25. Jarošová B, Erseková A, Hilscherová K, et al. Europe-wide survey of estrogenicity in wastewater treatment plant effluents: the need for the effect-based monitoring. Environ Sci Pollut Res Int 2014;21:10970-82.
26. Petrie B, Barden R, Kasprzyk-Hordern B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 2015;72:3-27.
27. Kase R, Javurkova B, Simon E, et al. Screening and risk management solutions for steroidal estrogens in surface and wastewater. TrAC Trends Anal Chem 2018;102:343-58.
28. Könemann S, Kase R, Simon E, et al. Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. TrAC Trends Anal Chem 2018;102:225-35.
29. Sonavane M, Schollée JE, Hidasi AO, et al. An integrative approach combining passive sampling, bioassays, and effect-directed analysis to assess the impact of wastewater effluent. Environ Toxicol Chem 2018;37:2079-88.
30. Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int J Androl 2008;31:233-40.
31. Carvalho RN, Ceriani L, Ippolito A, Lettieri T. Development of the first watch list under the environmental quality standards directive (Science and policy reports No. EUR 27142 EN). Available from: https://publications.jrc.ec.europa.eu/repository/handle/JRC95018. [Last accessed on 16 May 2023].
32. Liney KE, Hagger JA, Tyler CR, Depledge MH, Galloway TS, Jobling S. Health effects in fish of long-term exposure to effluents from wastewater treatment works. Environ Health Perspect 2006;114 Suppl 1:81-9.
33. Caldwell DJ, Mastrocco F, Anderson PD, Länge R, Sumpter JP. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ Toxicol Chem 2012;31:1396-406.
34. Brown AC, Stevenson LM, Leonard HM, Nieves-Puigdoller K, Clotfelter ED. Phytoestrogens β -sitosterol and genistein have limited effects on reproductive endpoints in a female fish, Betta splendens. Biomed Res Int 2014;2014:681396.
35. Bhandari RK, Wang X, Saal FSV, Tillitt DE. Transcriptome analysis of testis reveals the effects of developmental exposure to bisphenol a or 17α-ethinylestradiol in medaka (Oryzias latipes). Aquat Toxicol 2020;225:105553.
36. Anderson PD, Johnson AC, Pfeiffer D, et al. Endocrine disruption due to estrogens derived from humans predicted to be low in the majority of U.S. surface waters. Environ Toxicol Chem 2012;31:1407-15.
37. Tang Z, Liu ZH, Wang H, et al. Trace determination of eleven natural estrogens and insights from their occurrence in a municipal wastewater treatment plant and river water. Water Res 2020;182:115976.
38. Vandermarken T, Croes K, Van Langenhove K, et al. Endocrine activity in an urban river system and the biodegradation of estrogen-like endocrine disrupting chemicals through a bio-analytical approach using DRE- and ERE-CALUX bioassays. Chemosphere 2018;201:540-9.
39. Johnson AC, Dumont E, Williams RJ, Oldenkamp R, Cisowska I, Sumpter JP. Do concentrations of ethinylestradiol, estradiol, and diclofenac in European rivers exceed proposed EU environmental quality standards? Environ Sci Technol 2013;47:12297-304.
40. Connon RE, Geist J, Werner I. Effect-based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment. Sensors 2012;12:12741-71.
41. Escher BI, van Daele C, Dutt M, Tang JY, Altenburger R. Most oxidative stress response in water samples comes from unknown chemicals: the need for effect-based water quality trigger values. Environ Sci Technol 2013;47:7002-11.
42. Altenburger R, Ait-Aissa S, Antczak P, et al. Future water quality monitoring-adapting tools to deal with mixtures of pollutants in water resource management. Sci Total Environ 2015;512-513:540-51.
43. Wernersson A, Carere M, Maggi C, et al. The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ Sci Eur 2015:27.
44. European Commission, Fitness check of the water framework directive and floods directive. 2019. Available from: http://eeac.eu/wp-content/uploads/2020/01/2020-01-13_Council_Fitness-Check-Water_HS.pdf. [Last accessed on 22 May 2023].
45. European Commission, Technical report on aquatic effect-based monitoring tools. 2017. Available from: https://circabc.europa.eu/sd/a/0d78bbf7-76f0-43c1-8af2-6230436d759d/Effectbased%20tools%20CMEP%20report%20main%2028%20April%202014.pdf. [Last accessed on 16 May 2023].
46. Campbell CG, Borglin SE, Green FB, Grayson A, Wozei E, Stringfellow WT. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006;65:1265-80.
47. Guo W, Van Langenhove K, Vandermarken T, et al. In situ measurement of estrogenic activity in various aquatic systems using organic diffusive gradients in thin-film coupled with ERE-CALUX bioassay. Environ Int 2019;127:13-20.
48. Brion N, Verbanck MA, Bauwens W, Elskens M, Chen M, Servais P. Assessing the impacts of wastewater treatment implementation on the water quality of a small urban river over the past 40 years. Environ Sci Pollut Res Int 2015;22:12720-36.
49. Pfannkuche J, Schmidt A. Determination of suspended particulate matter concentration from turbidity measurements: particle size effects and calibration procedures. Hydrol Process 2003;17:1951-63.
50. Rogers JM, Denison MS. Recombinant cell bioassays for endocrine disruptors: development of a stably transfected human ovarian cell line for the detection of estrogenic and anti-estrogenic chemicals. In vitr Mol Toxicol ;2000 13:67-82.
51. Brennan JC, Bassal A, He G, Denison MS. Development of a recombinant human ovarian (BG1) cell line containing estrogen receptor α and β for improved detection of estrogenic/antiestrogenic chemicals. Environ Toxicol Chem 2016;35:91-100.
52. Elskens M, Baston DS, Stumpf C, et al. CALUX measurements: statistical inferences for the dose-response curve. Talanta 2011;85:1966-73.
53. Elwan A, Singh R, Patterson M, et al. Influence of sampling frequency and load calculation methods on quantification of annual river nutrient and suspended solids loads. Environ Monit Assess 2018;190:78.
54. Miège C, Choubert JM, Ribeiro L, Eusèbe M, Coquery M. Fate of pharmaceuticals and personal care products in wastewater treatment plants--conception of a database and first results. Environ Pollut 2009;157:1721-6.
55. Zhou Y, Zha J, Wang Z. Occurrence and fate of steroid estrogens in the largest wastewater treatment plant in Beijing, China. Environ Monit Assess 2012;184:6799-813.
56. Snyder SA, Westerhoff P, Yoon Y, Sedlak DL. Pharmaceuticals, personal care products, and endocrine disruptors in water: implications for the water industry. Environ Eng Sci 2003;20:449-69.
57. Liu YF, Lu G, Yin H, Dang Z, Rittmann B. Removal of natural estrogens and their conjugates in municipal wastewater treatment plants: A critical review. Environ Sci Tech :2015 49, 5288-5300.
58. Ting YF, Praveena SM. Sources, mechanisms, and fate of steroid estrogens in wastewater treatment plants: a mini review. Environ Monit Assess 2017;189:178.
59. Díaz-cruz M, García-galán M, Guerra P, et al. Analysis of selected emerging contaminants in sewage sludge. TrAC Trends Anal Chem 2009;28:1263-75.
60. Nieto A, Borrull F, Pocurull E, Marcé RM. Occurrence of pharmaceuticals and hormones in sewage sludge. Environ Toxicol Chem 2010;29:1484-9.
61. Avberšek M, Žegura B, Filipič M, Heath E. Integration of GC-MSD and ER-Calux® assay into a single protocol for determining steroid estrogens in environmental samples. Sci Total Environ 2011;409:5069-75.
62. Altenburger R, Brack W, Burgess RM, et al. Future water quality monitoring: improving the balance between exposure and toxicity assessments of real-world pollutant mixtures. Environ Sci Eur 2019:31.
63. Simon E, Duffek A, Stahl C, et al. Biological effect and chemical monitoring of Watch List substances in European surface waters: Steroidal estrogens and diclofenac- Effect-based methods for monitoring frameworks. Environ Int 2022;159:107033.
64. Vajda AM, Barber LB, Gray JL, Lopez EM, Woodling JD, Norris DO. Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ Sci Technol 2008;42:3407-14.
65. Barber LB, Rapp JL, Kandel C, et al. Integrated assessment of wastewater reuse, exposure risk, and fish endocrine disruption in the shenandoah river watershed. Environ Sci Technol 2019;53:3429-40.
66. Harraka GT, Magnuson JT, Du B, Wong CS, Maruya K, Schlenk D. Evaluating the estrogenicity of an effluent-dominated river in California, USA: Comparisons of
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.






















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].