fig7
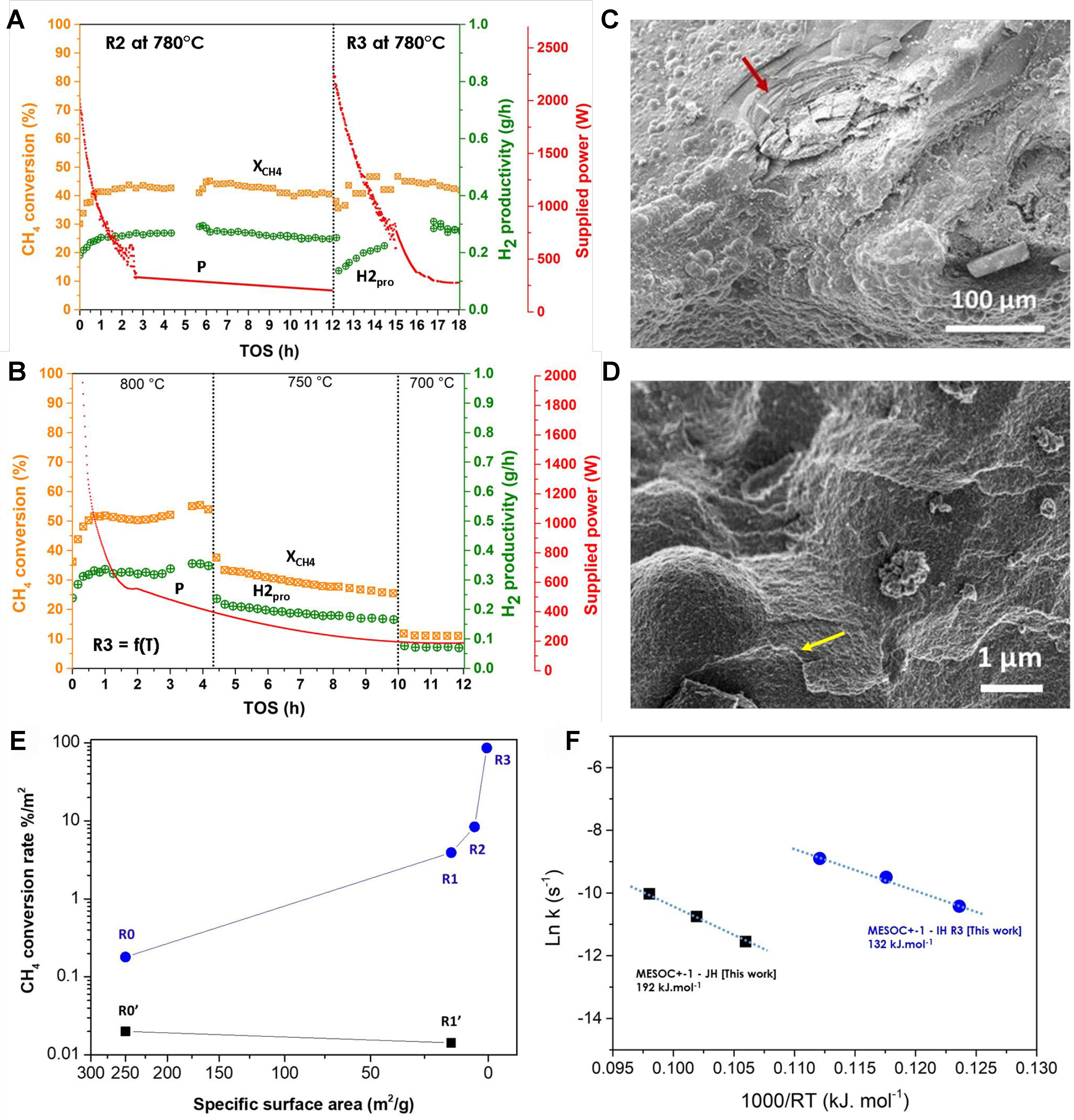
Figure 7. (A) CMD performance [CH4 conversion (XCH4), H2 productivity (H2pro), supplied power (P)] on the spent MESOC+ catalyst after two additional recycling tests (noted R1), at reaction temperature of 780 °C and for two consecutive cycling tests [spent catalyst labeled R2 (third cycling test) and R3 (fourth cycling test)]; (B) CMD performance [CH4 conversion (XCH4), H2 productivity (H2pro), supplied power (P)] on the spent R3 catalyst operated as a function of the reaction temperature ranged from 800 to 700 °C; (C and D) SEM micrographs of the R2 spent catalyst with bubbled carbon microspheres and graphene-like sheets deposited on the surface (indicated by arrows); (E) CMD performance, expressed in terms of CH4converted(%)/m2 (XCH4/m2) with respect to the SSA of the spent catalysts, operated under direct IH (blue) and indirect JH mode (black), showing the reverse relationship between the catalytic performance and the SSA values; (F) Comparison of the activation energy of the R3 catalyst calculated from the catalytic results obtained as a function of the reaction temperature under direct IH, i.e., 700, 750, and 800 °C, and that obtained on the pristine MESOC+ catalyst operated under indirect JH mode. CMD: Catalytic decomposition of methane; SEM: scanning electron microscopy; SSA: specific surface area; IH: induction heating; JH: Joule heating.








