fig14
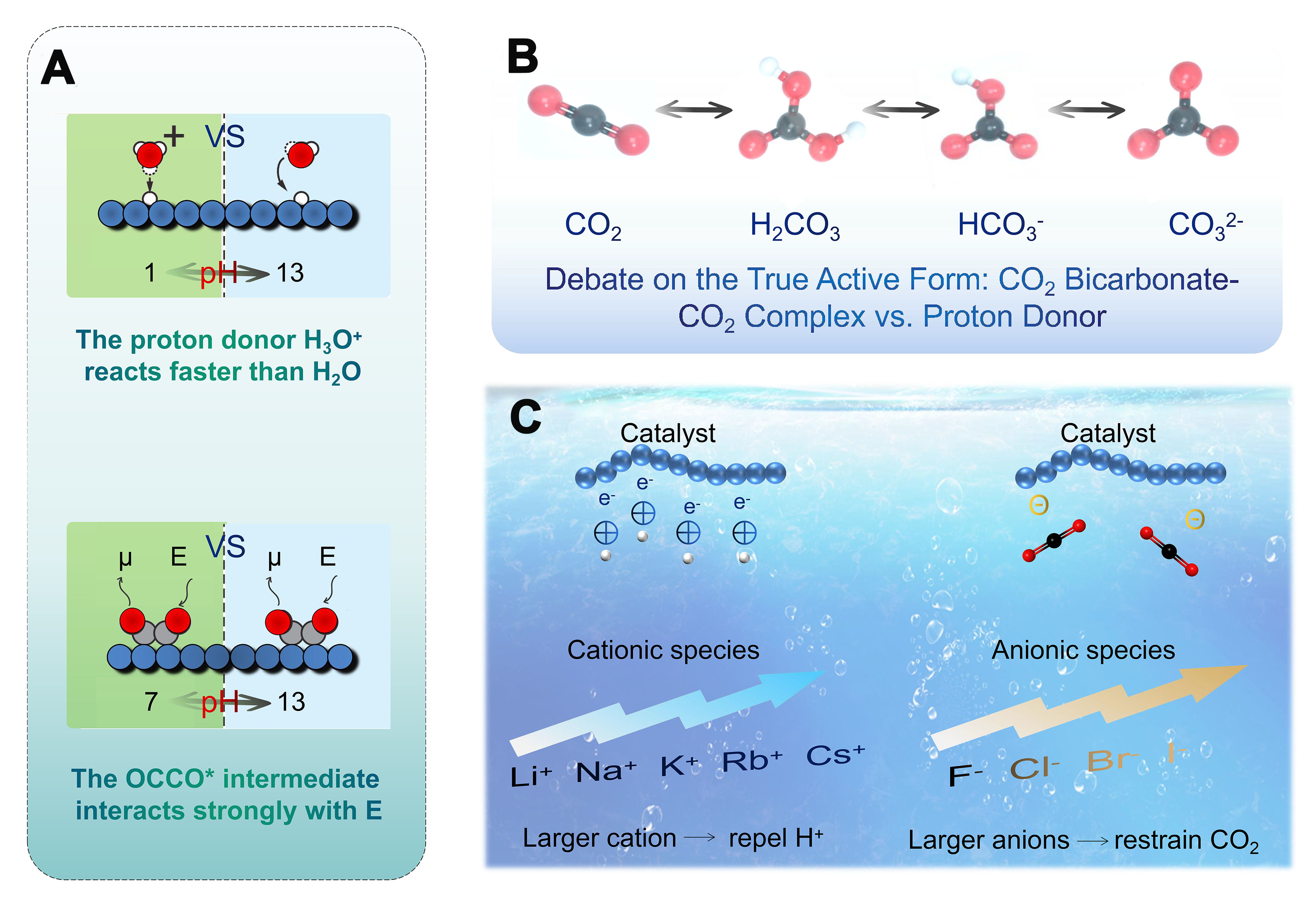
Figure 14. (A) The rate of electrocatalytic reactions can change with electrolyte pH primarily. Relative current densities are shown as bold numbers (Large electric fields E at high pH stabilize reactive intermediates with large dipole moments μ); (B) The discussion of CO2/H2CO3, H2CO3/HCO3-, and HCO3-/CO32- equilibria in aqueous solutions; (C) The cation and anion effects relating to the CO2 electroreduction.








