fig6
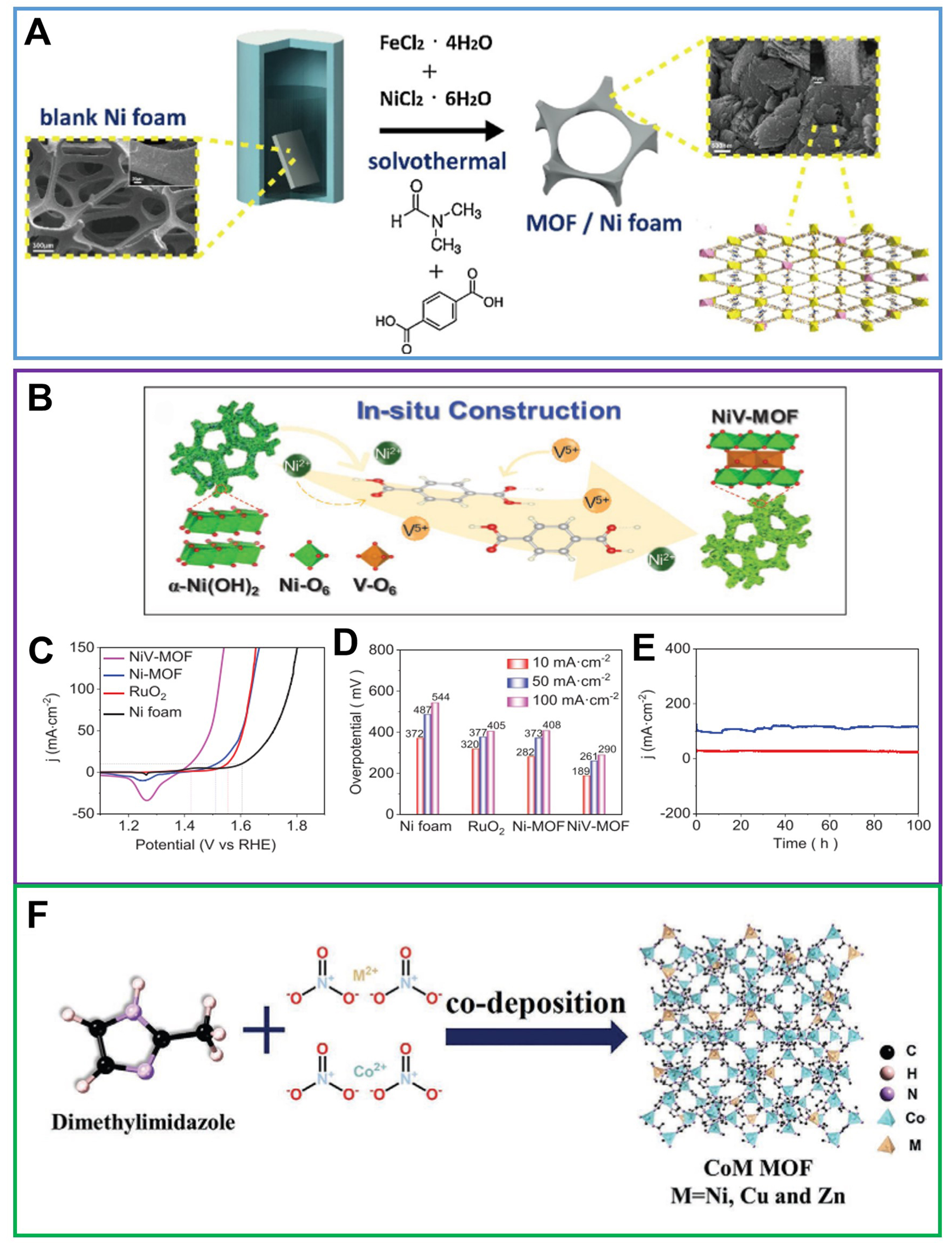
Figure 6. (A) Schematic illustration of synthetic process for one-step in situ growth of FeNi(BDC)(DMF,F) on NF, reproduced with permission[91]. Copyright 2019, Elsevier; (B) Schematic diagram of in situ construction of flexible V-Ni redox centers into NiV-MOF NAs; (C) LSV curves of NF, RuO2, Ni-MOF, and NiV-MOF NAs; (D) Comparison of the corresponding overpotentials at current densities of 10, 50, and 100 mA·cm-2; (E) Chronoamperometry test of NiV-MOF NAs measured at 1.46 and 1.52 V vs. RHE for 100 h. (B)-(E) were reproduced with permission[92]. Copyright 2021, Wiley-VCH; (F) Synthetic strategy of CoM MOFs/CC, reproduced with permission[94]. Copyright 2021, Wiley-VCH. BDC: 1,4-benzenedicarboxylate; DMF: N,N-dimethylformamide; NF: nickel foam; MOF: metal-organic framework; NAs: nanoarrays ; LSV: linear sweep voltammetry; RHE: reversible hydrogen electrode; CC: carbon cloth.








