fig5
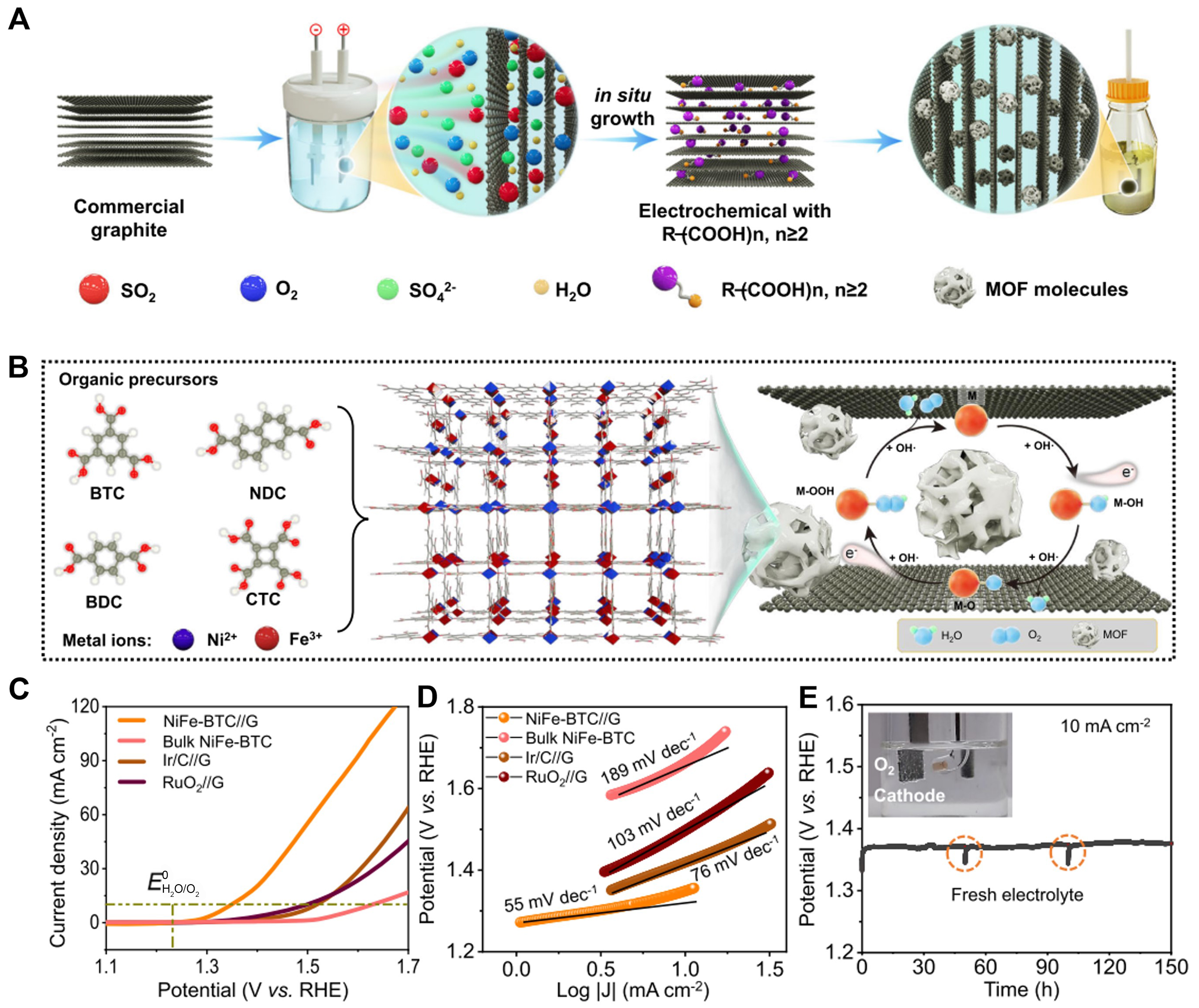
Figure 5. (A) Schematic illustration of the electrochemical synthesis process and (B) the resultant NiFe-MOF//G; (C) LSV plots obtained with NiFe-BTC//G, bulk NiFe-BTC, commercial Ir/RuO2 for OER at 10 mV·s-1 in 1.0 M KOH with Ag/AgCl as reference electrode; (D) Tafel plots obtained with NiFe-BTC//G, bulk NiFe-BTC, commercial IrO2 and RuO2; (E) Chronopotentiometric testing of NiFe-BTC//G for 150 h at 10 mA·cm-2 KOH. (A)-(E) were reproduced with permission[89]. Copyright 2022, Nature Publishing Group. MOF: Metal-organic framework; LSV: linear sweep voltammetry; BTC: 1,3,5-benzenetricarboxylic acid; OER: oxygen evolution reaction.








