fig17
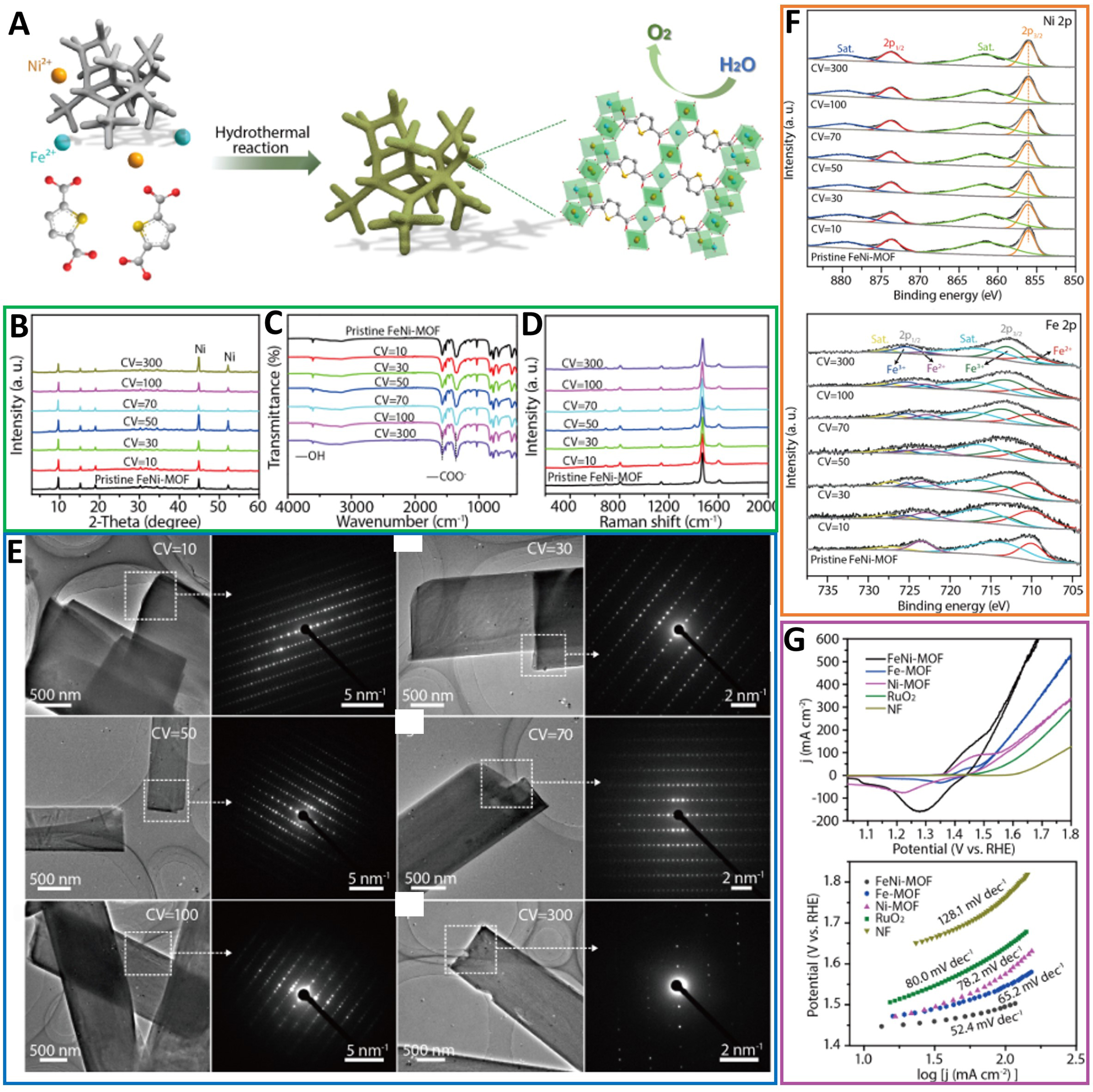
Figure 17. (A) Schematic illustration of synthesis of FeNi-MOF nanoarrays via a hydrothermal method. Structural characterizations of FeNi-MOF nanoarrays after various CV cycles; (B) XRD patterns and (C) FTIR and (D) Raman spectra of FeNi-MOF nanoarrays after different CV tests; (E) TEM images and the corresponding SAED patterns for FeNi-MOF nanobelts after different CV cycles; (F) Ni 2p and Fe 2p XPS spectra of FeNi-MOFs after different CV cycles; (G) Electrocatalytic properties of FeNi-MOF nanoarrays and other samples for OER and Tafel plots of different catalysts. CV curves toward OER at a scan rate of 5 mV·s-1. (A)-(G) were reproduced with permission[73]. Copyright 2021, American Chemical Society. MOF: Metal-organic framework; CV: cyclic voltammetry; XRD: X-ray diffraction; FTIR: Fourier transform infrared spectroscopy; TEM: transmission electron microscopy; SAED: selective area electron diffraction; XPS: X-ray photoelectron spectroscopy; OER: oxygen evolution reaction.








