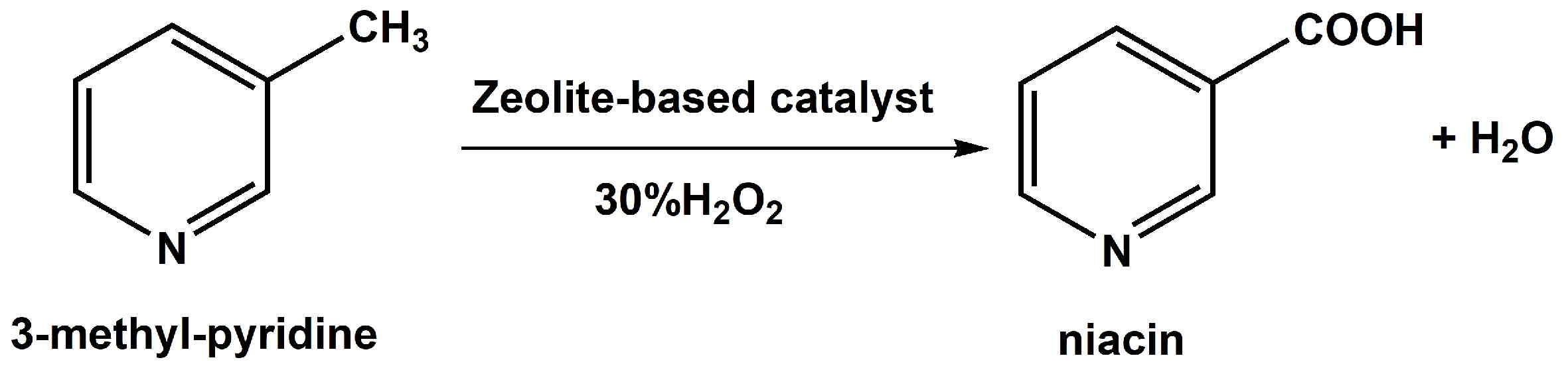
Catalytic synthesis of niacin from 3-methyl-pyridine and 30%H2O2 by Cu-based zeolite
Abstract
Niacin is well known not only as vitamin B3 but also as an important chemical, and has wide applications in the fields of food, farming, medicine, pharmaceuticals, and industry. With the rapid development of human society, the requirement for niacin has been increasing constantly worldwide. Meanwhile, development of green routes to produce niacin under mild conditions has become particularly urgent to substitute the conventional reaction process with the consideration of energy conservation and emission reduction. Among various synthesis routes of niacin, selective oxidation of 3-methyl-pyridine and hydrogen peroxide (H2O2) in the liquid phase has become the focus of research due to its distinct advantages, such as mild reaction conditions, environmentally friendly reaction processes, and so on. Herein, zeolite-based catalysts have been first applied in the liquid phase synthesis of niacin from 3-methyl-pyridine and 30%H2O2 under mild reaction conditions. In addition, Cu-based 13X zeolite is found to show the highest catalytic performance, and optimal catalytic reaction systems have been established. This work provides a green and optional route for the synthesis of niacin.
Keywords
INTRODUCTION
Niacin (nicotinic acid) is well known as the essential vitamin B3 for all living cells. People are prone to getting symptoms if they lack niacin as the human body does not produce it. Therefore, niacin is widely used as an additive for food and feed production and in medical drugs. Additionally, niacin has important applications in pharmaceutical synthesis and the chemical industry[1-7].
With the rapid development of human society, for example, the greatly increased demand for health, the global need for niacin has been increasing constantly. The conventional synthesis methods of niacin mainly include gas- (ammonia oxidation and air oxidation) and liquid-phase catalytic oxidation (inorganic acids as oxidants)[7-12]. However, the gas-phase oxidation faces several challenges, including harsh reaction conditions, complex and multi-step reaction processes, and high energy consumption. On the other hand, the liquid-phase oxidation encounters issues such as a corrosive production environment and enormous post-treatment pressure. Therefore, development of green routes to synthesize niacin under mild conditions has become an urgent task. In 2016, the selective oxidation of 3-methyl-pyridine to niacin was reported using Ag/Mn3O4 spinel nanorods as catalysts and 50%H2O2 as an oxidant[13]. On the one hand, this route offers several clear advantages, including mild reaction conditions, environmental friendliness, and fewer by-products. On the other hand, it sparks greater interest among researchers to develop new catalytic reaction systems considering economy and security, such as efficient non-noble metal-based catalysts, and low-concentrated H2O2.
Zeolite, an important crystalline material with unique and excellent physicochemical properties, is widely used in areas such as adsorption and separation, ion exchange, and catalysis[14-26]. Herein, a series of zeolite-based catalysts had been prepared and applied in the liquid phase synthesis of niacin from 3-methyl-pyridine and 30%H2O2 under mild reaction conditions [Scheme 1]. Unexpectedly, it was found that non-noble metal-based zeolites presented high catalytic activity, and the highest niacin yield was achieved over Cu-based 13X zeolite. In addition, the optimal catalytic reaction systems had been established based on the Cu-based 13X zeolite catalyst, including solvent type, metal content of catalyst, molar ratio of substrate and H2O2, oxidant type, reaction temperature and time.
EXPERIMENTAL
Chemicals and raw materials
The chemicals and raw materials used in the work included the ones purchased from Macklin [cupric sulfate anhydrous, CuSO4, 99%; copper nitrate trihydrate, Cu(NO3)2·3H2O, 99%; cobalt nitrate hexahydrate, Co(NO3)2·6H2O, 99%; copper acetate monohydrate, Cu(CH3COO)2·H2O, 99%], the ones from Aladdin [iron nitrate nonahydrate, Fe(NO3)3·9H2O, 98%; chromium nitrate nonahydrate, Cr(NO3)3·9H2O, 99%; 50% hydrogen peroxide aqueous solution, 50%H2O2; 3-methyl-pyridine, 99%; sodium hypochlorite solution aqueous, NaClO, 6%~14%], the ones from Sinopharm [zinc nitrate hexahydrate, Zn(NO3)2·6H2O, 99%; nickel nitrate hexahydrate, Ni(NO3)2·6H2O, 99%; silver nitrate, AgNO3, 99%; cuprous chloride, CuCl, 99%; acetonitrile, 99%; acetic acid, 99%; ethyl alcohol, 99%; acetone, 99%; tert-butyl hydroperoxide (TBHP) solution, 65%], the zeolites from Nankai University Catalyst Co LTD (Na-Y, Na-13X, Na-Beta, Na-4A, Na-MOR, Na-ZSM-5, TS-1), the one from Degussa (aerosil-200 fumed silica, SiO2, 99.9%), the one from Chengdu Kelong Chemical Co LTD (30% hydrogen peroxide aqueous solution, 30%H2O2), and self-made deionized water. All the above chemicals and raw materials were applied directly in the catalyst preparation and catalytic reaction without further purification.
Preparation of catalysts
A series of zeolite-based catalysts with the same metal content [Table 1], were prepared using different types of metal salts and zeolites based on the reported method[27], among which Cu/13X and Cu’/13X represented the catalysts prepared from cupric sulfate anhydrous and cuprous chloride, respectively. In addition, CuOx/SiO2 was also prepared for comparison through the following process. Materials 0.846 g Cu(NO3)2·3H2O,
Catalytic synthesis of niacin from 3-methyl-pyridine and 30%H2O2a
| Entry | Catalyst | Conversion of 3-methyl-pyridine (%) | Selectivity (%) | Yield of niacin (%) | ||
| Nicotinyl alcohol | Niacin | Others | ||||
| 1 | Zn/13X | 62.4 | 57.2 | 12.8 | 30.0 | 8.0 |
| 2 | Ag/13X | 93.3 | 68.1 | 15.0 | 16.9 | 14.0 |
| 3 | Cr/13X | 60.5 | 46.3 | 27.0 | 26.7 | 16.3 |
| 4 | Ni/13X | 63.7 | 55.6 | 30.2 | 14.2 | 19.2 |
| 5 | Co/13X | 65.8 | 49.3 | 36.2 | 14.5 | 23.8 |
| 6 | Fe/13X | 71.3 | 41.4 | 35.1 | 23.5 | 25.0 |
| 7 | Cu’/13X | 81.5 | 3.8 | 54.1 | 42.1 | 44.1 |
| 8 | Cu/13X | 83.6 | 2.3 | 69.0 | 28.7 | 57.7 |
| 9 | Cu/MOR | 64.9 | 6.4 | 55.2 | 38.4 | 35.8 |
| 10 | Cu/4A | 67.1 | 6.8 | 53.7 | 39.5 | 36.0 |
| 11 | Cu/ZSM-5 | 74.2 | 6.2 | 50.8 | 43.0 | 37.7 |
| 12 | Cu/Beta | 75.3 | 5.6 | 57.2 | 37.2 | 43.0 |
| 13 | Cu/Y | 80.1 | 5.4 | 61.6 | 33.0 | 49.3 |
| 14 | CuSO4 | 74.1 | 3.3 | 65.9 | 30.8 | 48.8 |
| 15b | Cu(NO3)2·3H2O | 75.4 | 3.3 | 61.8 | 34.9 | 46.6 |
| 16c | Cu(CH3COOH)2 | 74.3 | 3.1 | 65.0 | 31.9 | 48.3 |
| 17d | 13X | 94.5 | 81.9 | 7.2 | 10.9 | 6.8 |
| 18 | CuOx/SiO2 | 90.1 | 6.1 | 35.2 | 58.7 | 31.7 |
| 19 | TS-1 | > 99.9 | 97.8 | - | 2.2 | - |
| 20 | - | 99.1 | 94.3 | - | 5.7 | - |
Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a Bruker D8A25 diffractometer equipped with Cu Kα radiation (λ = 1.54184 Å) with a scanning speed of 10.0°/min. X-ray photoelectron spectroscopy (XPS) spectra were determined on a PerkinElmer PHI electron spectroscopy for chemical analysis (ESCA) system. Scanning electron microscopy (SEM) images were obtained on JEOL JSM-6510A and TheromoFisher Helios G4 CX, and energy dispersive X-ray spectroscopy (EDS) was performed using an Oxford Instrument Ultim Max 100. The textural parameters were measured on a Quantachrome iQ-MP gas adsorption analyzer by physical adsorption/desorption of nitrogen at 77 K. Before the nitrogen adsorption, samples were dehydrated at 523 K for 14 h. The surface area was calculated via the Brunauer-Emmett-Teller (BET) equation. The pore volume was obtained from the adsorbed amount of nitrogen at a relative pressure of approximately 0.98.
Catalytic reaction
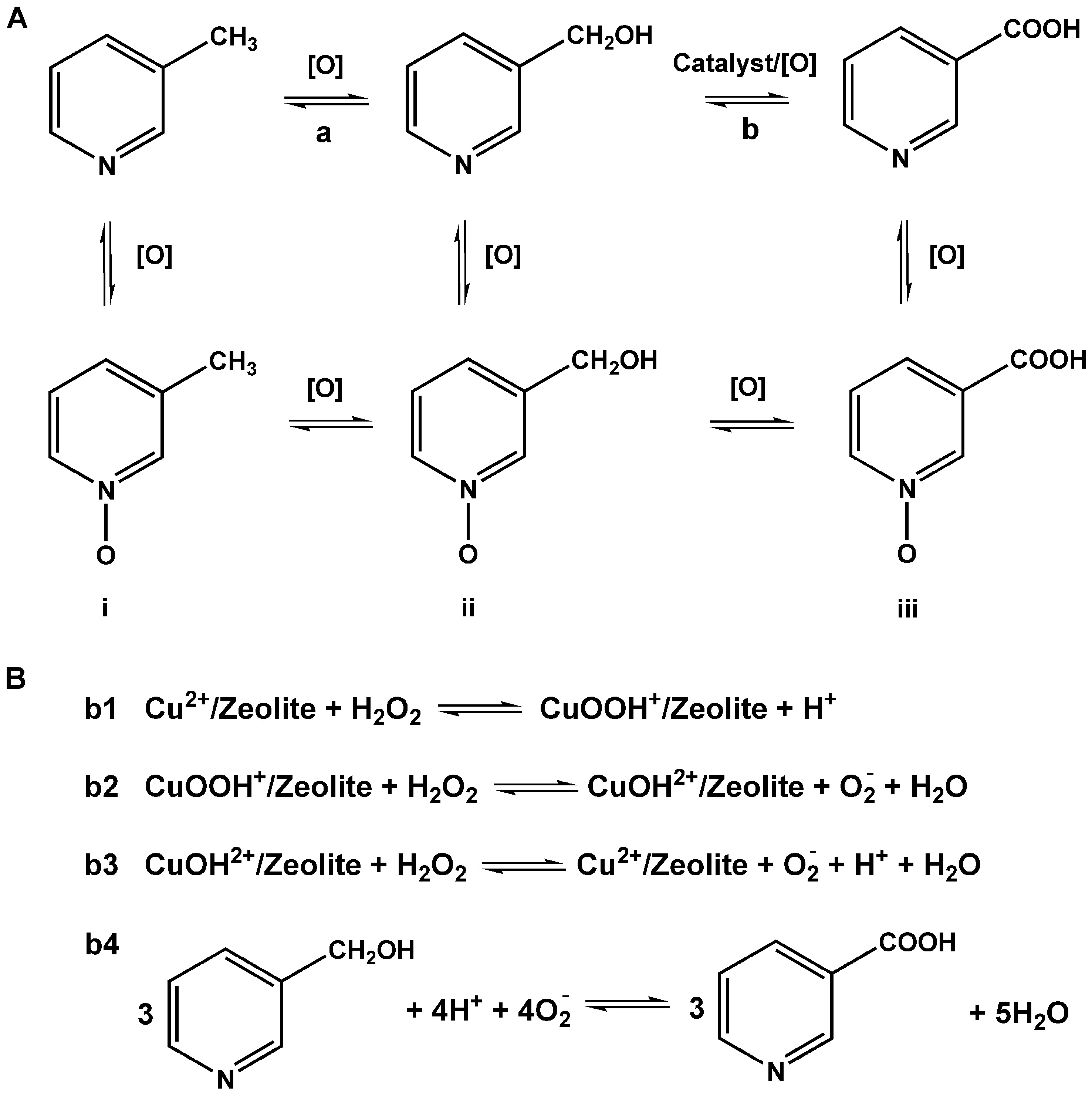
The catalytic reaction experiments were carried out under atmospheric pressure. Typically, 100 mg catalyst, 10 mL acetonitrile, and 10.7 mmol 3-methyl-pyridine were added successively into a 50 mL double-necked round-bottom flask equipped with a condensing device, and then the mixture was kept on stirring under a specific temperature for a period of time, during which aqueous solution of hydrogen peroxide was added into the mixture drop by drop. Subsequently, the reaction mixture was cooled to room temperature, and the liquid separated from the solid catalyst by centrifugation was analyzed by a liquid chromatography (LC, EClassical 3100 HPLC system, Dalian Elite Analytical Instruments CO LTD) equipped with a chromatographic column (O3100). The liquid chromatogram analysis results of 3-methyl-pyridine, niacin, and nicotinyl alcohol were shown in Supplementary Figures 1-3, respectively. In addition, an American Agilent 6224 time-of-flight liquid chromatography/mass spectrometry (TOF-LCMS) system was also applied to identify the products, and the analysis results were shown in Supplementary Figure 4. Based on the above analysis, “others” in Table 1 referred to the N-oxides of i and ii (entries 1 and 20) or the N-oxides of i, ii, and iii (entries 2~19) shown in Scheme 2. The conversion of 3-methyl-pyridine, selectivity of niacin, and yield of niacin were calculated based on: conversion of 3-methyl-pyridine (mol%) = (moles of 3-methyl-pyridine reacted/total moles of 3-methyl-pyridine added) × 100%, selectivity of niacin (%) = (moles of niacin formed/total moles of products) × 100%, yield of niacin (%) = (moles of niacin formed/total moles of 3-methyl-pyridine added) × 100%.
RESULTS AND DISCUSSION
Catalytic performance evaluations were conducted over various samples for the conversion of 3-methyl-pyridine with 30%H2O2 to niacin under the same reaction condition, and the results were listed in Table 1.
Entries 1~8 had compared the catalytic performance of various metal salt-based 13X zeolite catalysts. The yield of niacin over Zn/13X was merely 8.0%, which originated from low conversion of 3-methyl-pyridine (62.4%) and selectivity for niacin (12.8%). High conversion of 3-methyl-pyridine (93.3%) and quite low selectivity for niacin (15%) were obtained over Ag/13X. Although catalysts Cr/13X, Ni/13X, Co/13X, and Fe/13X showed distinctly increased selectivity for niacin (≥ 27%), the yields of niacin were not higher than 25%. In contrast, Cu’/13X achieved 81.5% conversion of 3-methyl-pyridine and 54.1% selectivity for niacin, and the yield of niacin reached 44%, distinctly higher than the above catalysts. For comparison, 83.6% conversion of 3-methyl-pyridine and 69.0% selectivity for niacin were acquired over catalyst Cu/13X, with a niacin yield of about 58%, obviously higher than that obtained over Cu’/13X. The above results indicated the efficiency of copper species in the synthesis of niacin.
Entries 8~13 had compared the effect of zeolite support on the catalytic performance of various Cu/zeolite catalysts prepared by cupric sulfate and different types of zeolites. It was found that the selectivity for niacin over various Cu/zeolite catalysts was higher than 50%, while the yield of niacin obtained with Cu/MOR, Cu/4A, Cu/ZSM-5, Cu/Beta and Cu/Y (< 50%) was significantly lower than that achieved with Cu/13X. In addition, different metal salts, including CuSO4, Cu(NO3)2·3H2O, and Cu(CH3COOH)2, had also been applied in the reaction directly (entries 14~16). It was found that these metal salts had shown similar conversion of 3-methyl-pyridine (74%~75%) and product distribution (about 62%~66% for niacin, 3% for nicotinyl alcohol, and 30%~32% for others), with the niacin yield distinctly lower than that of Cu/13X. When 13X parent zeolite was used as a catalyst, nicotinyl alcohol was the main product, and the selectivity for niacin was merely 7.2% (entry 17). In addition, when CuOx/SiO2 was applied in the reaction (entry 18), although 90.1% conversion of 3-methyl-pyridine was achieved, the yield for niacin was merely 31.7% due to its high selectivity for others (58.7%), implying its low catalytic activity for the reaction. The above results suggested the important roles of zeolite as support in the reaction, which influenced not only the conversion of 3-methyl-pyridine but also the distribution of products.
It was noteworthy that when TS-1 zeolite was applied in the reaction (entry 19), nearly all 3-methyl-pyridine was converted into nicotinyl alcohol, and no niacin was detected, indicating that TS-1 did not work in the selected conditions of this reaction. Interestingly, a similar result was observed in a blank experiment without a catalyst (entry 20). The above results implied that nicotinyl alcohol probably was an intermediate of the reaction. In order to prove this conjecture, a controlled experiment was particularly designed and conducted, in which the reaction proceeded under the system without a catalyst for the initial 4 h and then with a catalyst for the subsequent 2 h. Supplementary Figure 5 showed the product distributions corresponding to the fourth hour without a catalyst and the sixth hour with a catalyst. It was obvious that nicotinyl alcohol had been converted to niacin after the addition of a catalyst, which proved the above conjecture that nicotinyl alcohol was an intermediate of the reaction. The reusability tests of Cu/13X indicated that its catalytic activity did not decrease appreciably after reuse three times, implying its good chemical stability, and a slight decrease was ascribed to its tiny leaching of Cu2+ into the solution during the reaction. The XRD pattern and SEM image of the Cu/13X zeolite catalyst after reaction shown in Supplementary Figure 6 indicated its good structure stability.
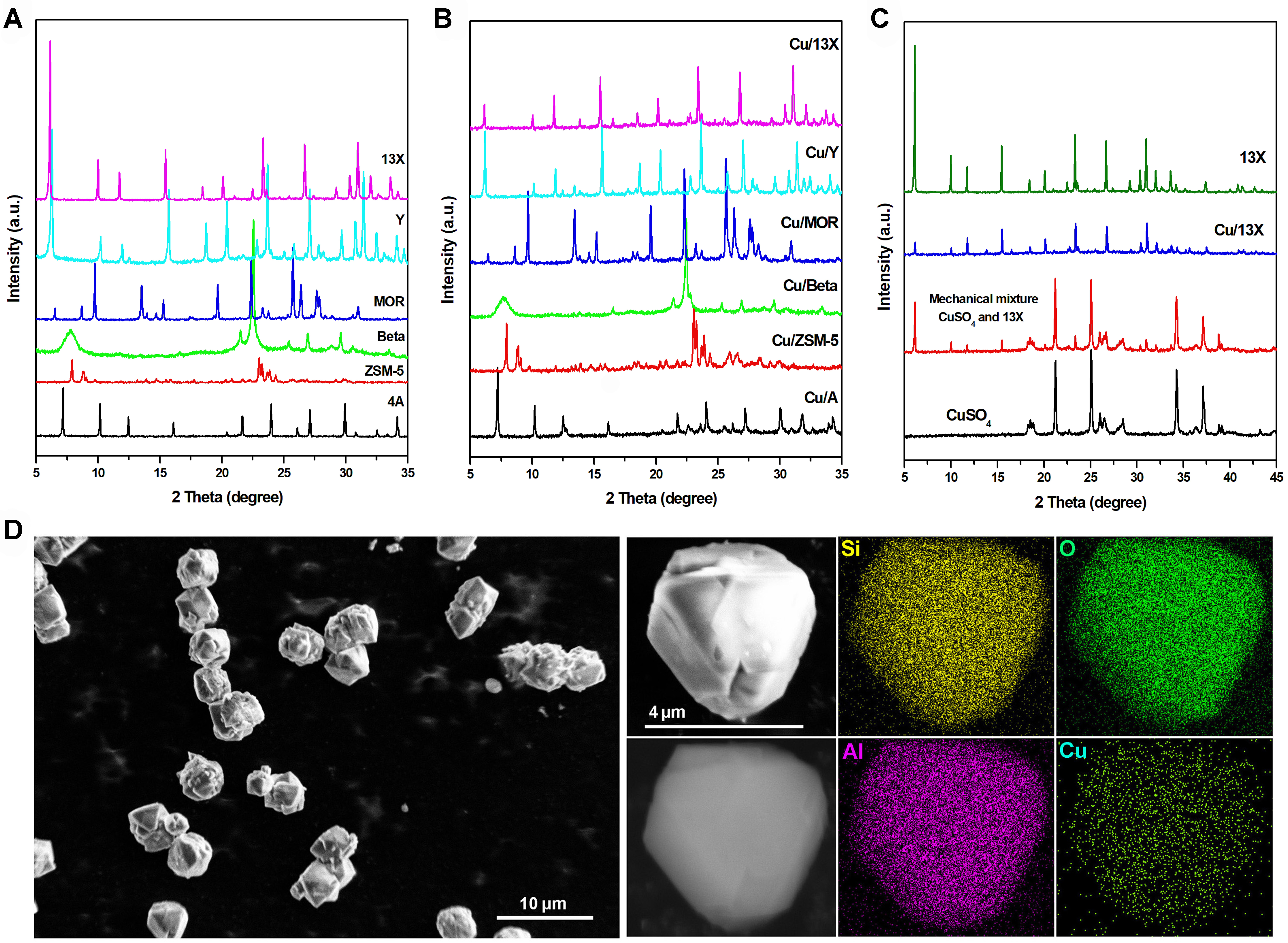
To explore the relationship between catalyst property and structure, various characterizations had been conducted over typical samples. The powder XRD patterns of selected samples were shown in Figure 1 and Supplementary Figure 7. Compared with the characteristic XRD patterns of parent zeolites, the counterparts of zeolite-based catalysts presented distinctly decreased XRD relative intensity, which was ascribed to the incorporation of copper species into the zeolites. Based on the XRD pattern of pure CuSO4 phase shown in Figure 1C, it was found that the peaks belonging to CuSO4 were obviously observed in the pattern of CuSO4/13X mechanical mixture but were extremely weak and nearly invisible in the patterns of zeolite-based catalysts. It suggested that the copper species had been highly dispersed over the zeolite support. The SEM and EDS of Cu/13X zeolite catalyst were shown in Figure 1D. It was observed that the particle size of Cu/13X zeolite catalyst was about 4.5 μm, and no obvious other phase was observed. The EDS images indicated that the distribution of various elements over the particle was highly uniform. This implied that the copper species had been highly dispersed over the 13X zeolite, consistent with the XRD analysis results.
Figure 1. (A-C) Powder XRD patterns of parent zeolites, zeolite-based catalysts, CuSO4, mechanical mixture of CuSO4 and 13X; (D) SEM and EDS of Cu/13X. XRD: X-ray diffraction; SEM: scanning electron microscopy; EDS: energy dispersive X-ray spectroscopy.
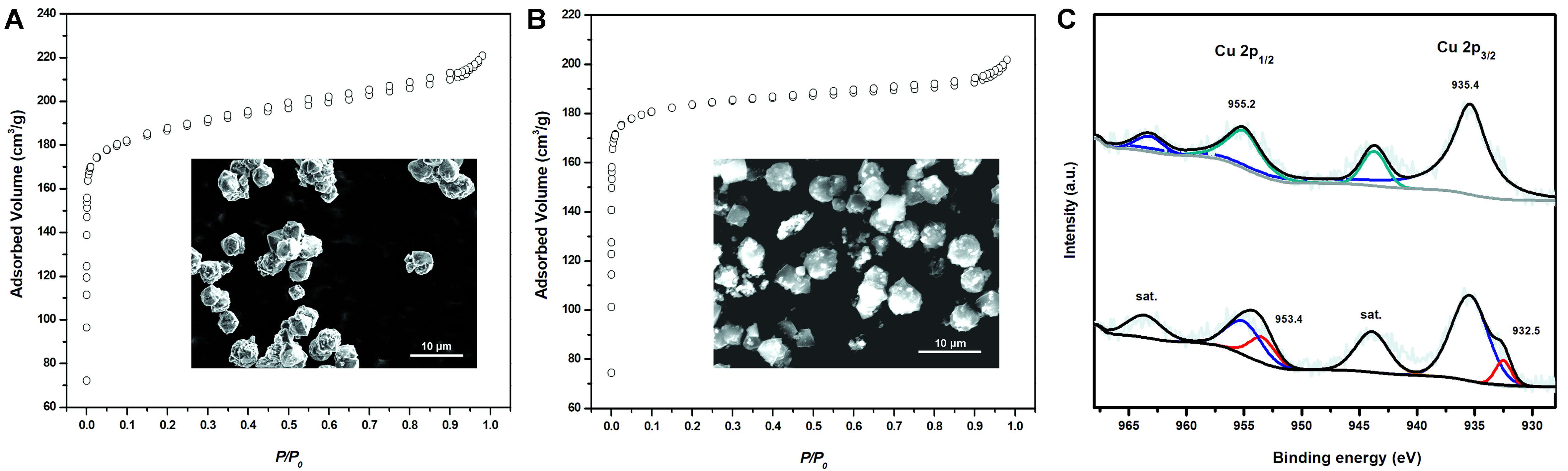
Particularly, based on the comparison of the SEM images [Figure 2], catalysts Cu/13X and Cu’/13X presented similar particle size and morphology, and no obvious difference was observed. In addition, the N2 adsorption-desorption isotherms of the two catalysts were also shown in Figure 2, which corresponded to a typical microporous structure. The textural parameters of selected zeolite-based catalysts were listed in Table 2. It was found that catalysts Cu/Y (779.8 m2/g), Cu/13X (746.2 m2/g), and Cu’/13X (743.7 m2/g) exhibited distinctly higher surface area than other catalysts (< 500 m2/g), and the average pore volume of catalysts Cu/13X (0.3128 m3/g) and Cu’/13X (0.2446 m3/g) were slightly higher than that of Cu/Y
Figure 2. SEM images, N2 adsorption-desorption isotherms, and XPS patterns of catalysts Cu/13X (A) and Cu’/13X (B); (C)top: Cu/13X, bottom: Cu’/13X. SEM: Scanning electron microscopy; XPS: X-ray photoelectron spectroscopy.
Textural parameters of Cu2+-exchanged zeolite catalysts
| Catalyst | Surface area (m2/g) | Pore volume (m3/g) |
| Cu/4A | 243.3 | 0.1308 |
| Cu/ZSM-5 | 327.5 | 0.1508 |
| Cu/Beta | 496.3 | 0.1844 |
| Cu/MOR | 388.5 | 0.2043 |
| Cu/Y | 779.8 | 0.1920 |
| Cu/13X | 746.2 | 0.3128 |
| Cu’/13X | 743.7 | 0.2446 |
The chemical states of Cu/13X and Cu’/13X were also characterized by XPS analysis [Figure 2]. In the XPS pattern of Cu/13X, the peaks attributed to Cu2p3/2 at 934.8 eV and Cu2p1/2 at 954.7 eV were observed, corresponding to Cu2+ states[28]. In the XPS pattern of Cu’/13X, peaks (Cu2p3/2 at 933.0 eV and Cu2p1/2 at 953.4 eV) for both Cu+ states and Cu2+ states were observed. This was ascribed to the partial conversion of Cu(I) to Cu(II) cations during the catalyst preparation process. Therefore, compared to catalyst Cu’/13X, the higher catalytic activity and selectivity to niacin displayed by Cu/13X was ascribed to its higher content of Cu(II) cations, based on the above reaction and characterization results. It also suggested the efficiency of Cu(II) cations as catalytic active sites in the conversion of 3-methyl-pyridine to niacin.
Based on the above results, the main steps in the reaction were shown in Scheme 2, including the first oxidation of 3-methyl-pyridine to nicotinyl alcohol by hydrogen peroxide [Scheme 2A] and then the oxidation of nicotinyl alcohol to niacin by zeolite-based catalysts [Scheme 2B]. The excellent catalytic performance shown by Cu/13X for the synthesis of niacin was ascribed to the cooperative contribution of Cu(II) ions and zeolite support, where Cu2+ ions played an important role in activating H2O2 molecules while the zeolite stabilized various intermediate ions in the reaction system.
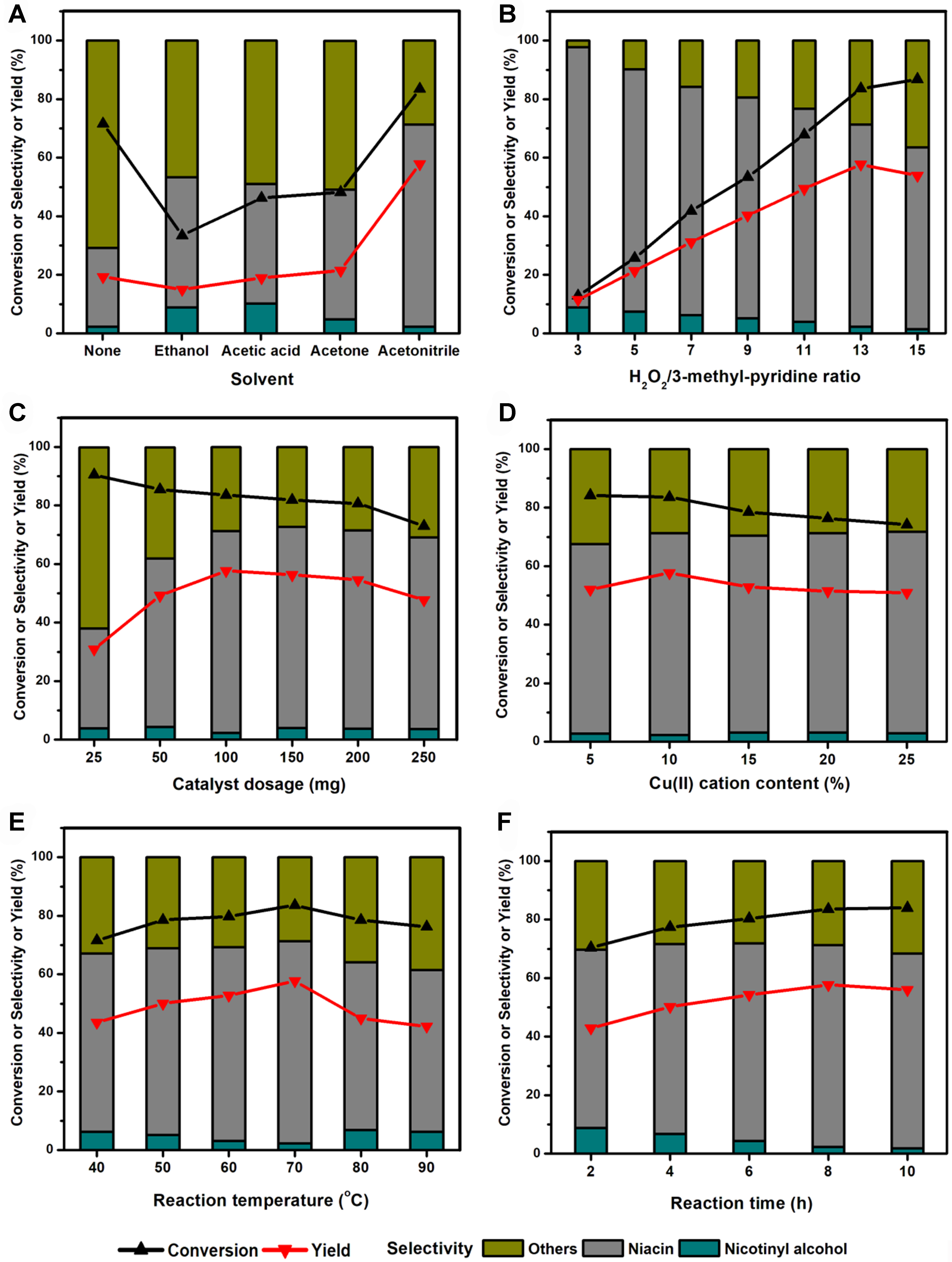
The effects of various parameters on the catalytic oxidation of 3-methyl-pyridine to niacin by using Cu/13X as a catalyst had been systematically studied, including the effects of solvent type, molar ratio of H2O2/3-methyl-pyridine, catalyst dosage, Cu(II) cation content of catalyst, reaction temperature, reaction time, and oxidant type, and the results were shown in Figure 3 and Table 3.
Figure 3. Effects of (A) solvent, (B) H2O2/3-methyl-pyridine ratio, (C) catalyst dosage, (D) metal content, (E) reaction temperature and (F) time.
Reaction results over 10%Cu/13X catalyst by using different oxidantsa
| Oxidant | Conversion of 3-methyl-pyridine (mol%) | Selectivity (mol%) | Yield of niacin (%) | ||
| Niacin | Nicotinyl alcohol | Others | |||
| 30%H2O2 | 83.6 | 69.0 | 2.3 | 28.7 | 57.7 |
| 50%H2O2 | 71.0 | 65.8 | 3.8 | 30.4 | 46.7 |
| TBHP | 33.7 | 58.8 | 20.8 | 20.4 | 19.8 |
| NaClO | 34.2 | 49.4 | 26.2 | 24.2 | 16.9 |
| Airb | - | - | - | - | - |
| None | - | - | - | - | - |
By using 10%Cu/13X as a catalyst, the effect of solvent type on the reaction was first explored [Figure 3A], including polar solvents ethanol, acetone, acetic acid, and acetonitrile. When no organic solvent was added into the reaction system, the reaction happened in the environment of water that was introduced by
The effect of H2O2/3-methyl-pyridine molar ratio on the reaction over 10%Cu/13X was shown in Figure 3B. With the increase of H2O2/3-methyl-pyridine molar ratio from 3 to 13, the yield of niacin had increased distinctly from 11.5% to 57.7%, which corresponded to the sharply increased conversion of 3-methyl-pyridine from 12.9% to 86.8% and slightly decreased selectivity for niacin from 88.8% to 69.0%. Based on the product distribution data, a decrease in selectivity of niacin was accompanied by a gradual reduction in nicotinyl alcohol and an increase in other by-products. With the rise of H2O2/3-methyl-pyridine molar ratio from 13 to 15, although the conversion of 3-methyl-pyridine had continuously increased from 83.6% to 86.8%, the yield of niacin had decreased from 57.7% to 53.9%. It was mainly ascribed to the sharply decreased selectivity of niacin from 69.0% to 62.1%.
Figure 3C had compared the effect of catalyst dosage on the reaction. It was found that with the increase of 10%Cu/13X catalyst dosage from 25 to 250 mg, the conversion had declined gradually from 90.5% to 73.0%, and the yield of niacin reached the maximum value when 100 mg catalyst was used. When the catalyst dosage of 10%Cu/13X increased from 25 to 100 mg, the yield of niacin grew gradually, which was led by the slightly decreased conversion from 90.5% to 83.6% and distinctly increased selectivity from 34.1% to 69.0%. If catalyst dosage was further increased from 100 to 250 mg, the yield showed a slight reduction, which was led by a distinct decline in conversion from 83.6% to 73.0% and a slight drop in selectivity from 69.0% to 65.4%. The phenomenon where the conversion of 3-methyl-pyridine decreased with increasing catalyst amount was ascribed to two main respects. First, the catalyst played the catalytic roles on both the forward and reverse reactions, which was assumed to be the primary reason when the catalyst dosage was relatively low. Second, partial decomposition of hydrogen peroxide induced by the elevated catalyst became the main reason when the catalyst dosage was relatively high.
By the same preparation process except different Cu(II) cation contents in theory, a series of Cu/13X catalysts had been prepared and applied in the reaction, and the results were shown in Figure 3D. It was found that, as Cu(II) cation contents increased from 5% to 25%, the conversion had decreased slightly from 83.6% to 74.2%. First, this was attributed to the limited actual supporting amount of CuSO4 over 13X zeolite; second, to the partial decomposition of hydrogen peroxide caused by the excessive Cu species over the catalyst. The highest yield of niacin was achieved over catalyst 10%Cu/13X.
Figure 3E had compared the effect of temperature on the reaction, which was positive when the temperature was lower than 70 °C but negative when higher than 70 °C. The phenomenon where the conversion of 3-methyl-pyridine decreases with increasing reaction temperature from 70 to 90 °C was mainly ascribed to the accelerated decomposition of hydrogen peroxide induced by the increased temperature. For example, the yield of niacin decreased greatly to 42.2% under a reaction temperature of 90 °C, corresponding to 76.3% conversion and 55.3% selectivity. The yield of niacin reached the maximum value of 57.7% under 70 °C, which was determined to be the optional reaction temperature.
Figure 3F demonstrated the effect of time on the reaction. As the reaction time extended from 2 to 8 h, both the conversion and selectivity improved gradually, corresponding to the yield rise from 42.9% to 57.7%. When the time was continuously prolonged to 10 h, it was found that the conversion had maintained at about 84%, but the by-products had increased distinctly, and the yield of niacin showed a slight decrease trend to 56%. It was probably due to the nearly complete consumption of H2O2 in 8 h, and the consequent reaction had accelerated the formation of by-products.
The effect of oxidant type on the reaction was shown in Table 3. It was found that the yield of niacin had obviously decreased when 30%H2O2 was substituted by 50%H2O2 under the same reaction condition, mainly due to the obvious decrease in conversion and selectivity. TBHP and NaClO were unsuitable oxidants for the selective oxidation of 3-methyl-pyridine, and the yield of niacin was lower than 20%. When air was introduced into the reaction system as an oxidant, the reaction did not occur, consistent with the result that no oxidant was used, implying the ineffectiveness of air and the necessity of 30%H2O2 as an oxidant.
Based on the above results and demonstration, obvious advantages were displayed by Cu-based 13X zeolite catalysts in the liquid phase synthesis of niacin from 3-methyl-pyridine and hydrogen peroxide under mild reaction conditions compared with the reported work[13], including simple and low cost of catalyst preparation processes, low concentration of hydrogen peroxide, high yield of niacin, and more
CONCLUSIONS
The Cu/13X zeolite catalyst has been prepared and applied in the selective oxidation of 3-methyl-pyridine and 30%H2O2 to synthesize niacin under mild conditions, demonstrating highly efficient catalysis activity and selectivity. This is mainly ascribed to the highly dispersed Cu(II) ions and their co-contribution with the zeolite support. The optimal reaction conditions indicate that this is a green reaction process for the synthesis of niacin with potential applications.
DECLARATIONS
Authors’ contributions
Carried out the experiments, analyzed the data, and wrote the draft manuscript: Liu Z, Shuai J
Analyzed the data: Xu W, Lu X
Provided helpful discussion: Xia Q
Directed and supervised the project, analyzed the data, and wrote and revised the manuscript: Zhou D
Availability of data and materials
The data and materials supporting the findings of this study are available within the article and its Supplementary Materials.
Financial support and sponsorship
The authors thank the financial support from the National Natural Science Foundation of China (22272045, U20A20122, 22072038).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
1. Freese R, Lysne V. Niacin - a scoping review for Nordic Nutrition Recommendations 2023. Food Nutr Res. 2023;67.
2. Ahmadian A, Bouyeh M, Seidavi AR. A review of the effects of niacin on broiler productivity. World Poultry Sci J. 2021;77:589-604.
3. Rennick A, Kalakeche R, Seel L, Shepler B. Nicotinic acid and nicotinamide: a review of their use for hyperphosphatemia in dialysis patients. Pharmacotherapy. 2013;33:683-90.
4. Lanska DJ. The discovery of niacin, biotin, and pantothenic acid. Ann Nutr Metab. 2012;61:246-53.
5. Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the central nervous system: an update of biological aspects and clinical applications. Int J Mol Sci. 2019;20:974.
6. Song Z, Wang G, Li W, Li S. Innate pharmacophore assisted selective C–H functionalization to therapeutically important nicotinamides. Org Chem Front. 2019;6:1613-8.
7. Lisicki D, Nowak K, Orlińska B. Methods to produce nicotinic acid with potential industrial applications. Materials. 2022;15:765.
8. Lisicki D, Talik D, Orlińska B. Oxidation of picoline with oxygen to nicotinic acid against Co2+, NHPI, and phosphonium or ammonium bromides. Catalysts. 2023;13:1271.
9. Goto Y, Shimizu K, Murayama T, Ueda W. Hydrothermal synthesis of microporous W–V–O as an efficient catalyst for ammoxidation of 3-picoline. Appl Catal A Gen. 2016;509:118-22.
10. Jiang F, Deng S, Niu L, Xiao G. Effect of supports on the structure and activity of vanadium-chromium oxide catalysts for ammoxidation of 3-picoline. Chinese J Catal. 2013;34:1833-8.
11. Kalevaru VN, David Raju B, Venkat Rao V, Martin A. Preparation, characterisation and catalytic evaluation of MgF2 supported V2O5 catalysts for ammoxidation of 3-picoline. Appl Catal A Gen. 2009;352:223-33.
13. Ghosh S, Acharyya SS, Sharma SK, Bal R. Fabrication of Ag/Mn3O4 nano-architectures for the one-step selective oxidation of 3-picoline to niacin: a key to vitamin B3 production. Catal Sci Technol. 2016;6:4644-54.
14. Gao ZR, Yu H, Chen FJ, et al. Interchain-expanded extra-large-pore zeolites. Nature. 2024;628:99-103.
15. Liu L, Lu J, Yang Y, et al. Dealuminated Beta zeolite reverses Ostwald ripening for durable copper nanoparticle catalysts. Science. 2024;383:94-101.
16. Yu S, Liu Z, Lyu JM, et al. Engineering surface framework TiO6 single sites for unprecedented deep oxidative desulfurization. Natl Sci Rev. 2024;11:nwae085.
17. Liu S, Lian X, Yue B, et al. Control of zeolite local polarity toward efficient xenon/krypton separation. J Am Chem Soc. 2024;146:8335-42.
18. Gong X, Tuo J, Wang J, et al. Hydrophilic Ti-MWW for catalyzing epoxidation of allyl alcohol. Chem Synth. 2024;4:14.
19. Tai W, Dai W, Wu G, Li L. A simple strategy for synthesis of b-axis-oriented MFI zeolite macro-nanosheets. Chem Synth. 2023;3:38.
20. Sun MH, Gao SS, Hu ZY, et al. Boosting molecular diffusion following the generalized Murray’s Law by constructing hierarchical zeolites for maximized catalytic activity. Natl Sci Rev. 2022;9:nwac236.
21. Liu X, Liu H, Chen L, et al. Construction of Ti-containing zeolite with highly enhanced catalytic activity by active species surface implanting strategy. Catal Today. 2022;405-6:285-98.
22. Wang J, Zhang X, Chen L, Lu X, Xia Q, Zhou D. A nucleation-tuned mechanism to prepare centre-crossed zeolite lamellas by the rotating/static switch crystallization strategy. Inorg Chem Front. 2022;9:889-901.
23. Chai Y, Dai W, Wu G, Guan N, Li L. Confinement in a zeolite and zeolite catalysis. Acc Chem Res. 2021;54:2894-904.
24. Chen LH, Sun MH, Wang Z, Yang W, Xie Z, Su BL. Hierarchically structured zeolites: from design to application. Chem Rev. 2020;120:11194-294.
25. Hu N, Li X, Liu S, et al. Enhanced stability of highly-dispersed copper catalyst supported by hierarchically porous carbon for long term selective hydrogenation. Chinese J Catal. 2020;41:1081-90.
26. Peng Z, Chen L, Sun M, et al. A hierarchical zeolitic Murray material with a mass transfer advantage promotes catalytic efficiency improvement. Inorg Chem Front. 2018;5:2829-35.
27. Zhou D, Tang B, Lu X, Wei X, Li K, Xia Q. Co2+-exchanged MOR and 5A zeolites as efficient solid catalysts for the epoxidation of styrene with air. Catal Commun. 2014;45:124-8.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].