The effect of accessibility to acid sites in Y zeolites on ring opening reaction in light cycle oil hydrocracking
Abstract
The light cycle oil (LCO) hydrocracking process converts polycyclic aromatics into highly valuable light aromatics such as benzene, toluene, xylene (BTX), in accordance with the requirements of low-carbon development and high-quality transformation from oil refining to chemical industry. The accessibility of acid sites is a critical factor that impacts LCO conversion and BTX yield. Initially, the fine structure and molecular size of the typical polycyclic aromatics in LCO and their hydrogenation reaction intermediates were investigated through gas chromatography-mass spectrometry (GC-MS) analysis and density functional theory (DFT) calculations. Three porous Y zeolites with comparable Si/Al molar atomic ratios and pyridine (Py)-measured Brønsted acid amounts were chosen as an acidic component to prepare NiMo/(Al2O3 + HY) catalysts. The acid accessibility of HY zeolite was characterized via dual-beam infrared spectroscopy using 2,4,6-tri-tert-butylpyridine (2,4,6-TTBPy) and trihexylamine (THA) as probe molecules, and the LCO hydrocracking performance was evaluated on a fixed-bed reactor. The results revealed that bicyclic aromatic hydrocarbons featuring multiple, short side chains such as dimethylnaphthalene and trimethylnaphthalene are the main components of LCO, with sizes larger than those of HY zeolite micropores. There is a strong positive correlation between LCO conversion and ring-opening rate of polycyclic aromatics and cycloalkanes in LCO. Among these three HY zeolite catalysts, the ring-opening rates of polycyclic aromatics and cycloalkanes, and the yields of C6-C12 aromatics and BTX increased in the order of HY1 < HY2 < HY3, which is consistent with external surface acidity measured by THA as the probe molecule.
Keywords
INTRODUCTION
Light cycle oil (LCO) is a main byproduct derived from the fluid catalytic cracking (FCC) process, known for its characteristic features including high density, elevated levels of sulfur and nitrogen impurities coupled with a low cetane number. With up to 90% total aromatic content comprising mainly bicyclic and tricyclic polycyclic[1-4], LCO has been utilized as a blending component for diesel production through processes such as hydro-refining or hydro-upgrading technologies. However, growing environmental and health regulations concerning polycyclic aromatic hydrocarbon (PAH) content in clean fuels have become more stringent. Currently, the Euro VI Emission Standard limits it to no more than 8% (m/m), while the China VI Vehicle Emission Standard sets an even lower maximum at 7% (m/m). Anticipated further tightening of these restrictions has sparked increased interest in the utilization of LCO through hydrocracking processes to produce highly valuable chemicals such as benzene, toluene, xylene (BTX)[2,3,5-14].
Under the hydrocracking conditions [Figure 1], LCO initially undergoes hydrogenation saturation reactions of PAHs and the removal of sulfur and nitrogen impurities to obtain hydrotreated LCO (HDT-LCO), which is enriched in tetralin-based monocyclic aromatics hydrocarbons. It then enters the hydrocracking reaction zone where the tetralin-based monocyclic aromatic hydrocarbons experience ring-opening and cracking tandem reaction, yielding BTX. The ring-opening involving tetralin-based monocyclic aromatics hydrocarbons is a crucial and essential process step that enhances LCO conversion and BTX selectivity during this process.
Figure 1. The simplified reaction process of LCO hydrocracking to produce BTX. LCO: Light cycle oil; BTX: benzene, toluene, xylene.
In the presence of traditional transition metal sulfide-zeolite bifunctional hydrocracking catalysts, the ring-opening reaction of tetralin-based monocyclic aromatics is typically catalyzed by Brønsted acid sites (BAS) located on zeolite[15,16]. Owing to its suitable pore structure and adjustable acidity, HY zeolite is commonly utilized as a catalyst acidic component for LCO hydrocracking in both industry and academic research. The quantity, strength, density, and accessibility of BAS on HY zeolite are primary factors affecting the activity and selectivity of ring-opening reaction[17]. Due to the small dimensions of HY zeolite micropores
In this study, the chemical composition and structure of typical PAHs in LCO and their partially hydrogen-saturated products in HDT-LCO were initially determined at the molecular level and then the hydrocracking process was studied through a fixed-bed high-throughput reactor. Simultaneously, three hierarchical porous HY zeolites with similar Si/Al molar atomic ratios and total Brønsted amounts (measured by Py as probe molecule) were chosen as acidic components of catalysts. The acidities of HY zeolites were characterized through a dual-beam FTIR (DB-FTIR) with three alkaline probe molecules molecule: Py, 2,4,6-tri-tert-butylpyridine (2,4,6-TTBPy) and trihexylamine (THA), each possessing different molecular sizes and configuration. Based on DB-FTIR and density functional theory (DFT) calculation results, the accessibility of acid sites in the three HY zeolites was compared, and the effects of acid accessibilities on ring-opening performance of PAHs and BTX yields during LCO hydrocracking were also investigated. The assessment of acid accessibility, adapted to the size and adsorption states of reactant molecules, is essential for precise design and development of high-performance zeolite catalytic materials and hydrocracking catalysts.
EXPERIMENTAL
LCO and HDT-LCO characterization
To obtain the molecular size of aromatic hydrocarbons in LCO and HDT-LCO, hydrocarbon composition and carbon number distribution were analyzed in detail. The hydrocarbon composition is measured by standard methods ASTM D 2425 and ASTM D 8276 with Agilent 7890A/MSD5977 gas chromatography-mass spectrometry (GC-MS). The carbon number distribution of hydrocarbons is measured by JEOL JMS-T200GC-TOF gas chromatography-field ionization source time of fight mass spectrometry (GC-FI TOFMS). A 0.2 μL sample was injected onto a HP-5 MS column (30 m × 0.25 mm inner diameter × 0.25 μm film thickness), which was held at 60 °C for 2 min, ramped to 300 °C at 20 °C/min, and then held at a final temperature for 5 min. The GC-TOF MS interface temperature was at 300 °C. The field ionization emitter was at 10 kV voltage, with a 10 mA emitter current on a 10 μm emitter.
Catalyst preparation
Three hierarchical porous HY zeolites with similar Si/Al molar atomic ratios but different pore distributions, denoted as HY1, HY2, and HY3, were selected as acidic components of catalysts
Subsequently, a composite carrier of zeolite and alumina with a dry mass ratio of 60 wt% HY zeolite and 40 wt% binder was prepared as follows: the HY zeolite mixed with pseudo-boehmite (Sasol Co., Germany) and homogenized, and then extruded using a single-screw extruder (the BONNOT Co., America) through a die with a diameter of 1.6 mm. The resulting extrudates were dried at 393 K for 3h followed by calcination at 873 K under an air atmosphere for an additional 3 h.
Finally, the NiMo/(HY-Al2O3) catalysts were prepared by impregnating molybdenum trioxide, nickel carbonate hydroxide, and phosphoric acid aqueous solution onto the supports with 15 wt% MoO3, 3 wt% NiO and 3 wt% P2O5. Subsequently, they were dried at 393 K for 3 h and calcined at 723 K under an air atmosphere for another 3 h to obtain corresponding catalysts labeled as C1, C2, and C3 [Supplementary Table 2].
Zeolites and catalysts characterization
The crystal structures of zeolite were characterized through X-ray diffraction (XRD) using a German Siemens D5005 X-ray diffractometer, with Cu Kα radiation source and a scanning range of 2θ from 4° to 36°, at a tube voltage of 40 kV and tube current of 40 mA.
The specific surface areas and pore size distributions were determined by N2 adsorption-desorption isotherms at 77 K, performed on an ASAP 2400 automatic adsorption analyzer produced by Micromeritics. Zeolite samples (20-40 mesh) were first vacuum-treated at 350 °C for 4 h. N2 was used as the adsorbate, and adsorption was performed at -196 °C until reaching a state of static adsorption equilibrium. The specific surface area of the molecular sieve was calculated using the Brunauer-Emmett-Teller (BET) equation, and the pore size distribution was determined using the Barrett-Joyner-Halenda (BJH) model.
The aluminum distribution of the zeolites was obtained by using 27Al magic angle spinning nuclear magnetic resonance (MAS NMR) on a Bruker Avance 500 NMR spectrometer.
A DB-FTIR using the Nicolet IS 50 FTIR spectrometer was employed to qualitatively characterize the acidic sites of the zeolites by the adsorption of alkaline probe molecules Py, 2,4,6-TTBPy and THA. The zeolite powder was pressed into a self-supporting thin wafer (1.5 cm2), which was placed in the sample beam of the DB-FTIR; the reference beam was not placed with the sample. Then, the sample was decontaminated at
Performance evaluation
The catalyst performance evaluation was conducted on a 16-channel fixed-bed high-throughput evaluation equipment with HDT-LCO containing 1,000 ppm S and 500 ppm N as feedstock. Typically, a 2 mL catalyst (16-20 mesh) was loaded in a tubular reactor, and inert SiO2 (40-60 mesh) was filled on both sides of the reactor constant temperature zone. The catalyst needs to be pre-sulfurized before the reaction, using kerosene containing 2.5% dimethyl disulfide (DMDS) as the sulfurization oil at 573 K and 6.4 MPa for 8 h. After sulfurization, switch to the reaction oil and adjust the reaction pressure to 5.5 MPa, and raise the temperature to reaction temperature in the range of 663 to 683 K. After stabilizing for 48 h, the gas products were quantitatively analyzed using online gas chromatography, and the liquid products were analyzed off-line.
The LCO conversion and ring-opening rate were determined by
The conversion of LCO:
The ring-opening rate of PAHs and polycyclic cycloalkanes in LCO:
where min and mout refer to the mass fractions of the components in the feedstock and products with boiling point higher than 478 K, respectively. Cin and Cout refer to the total amounts of cycloalkanes and aromatic hydrocarbons containing two or more rings in the feedstock and products, respectively. It is worth noting that during the hydrocracking process, tricyclic aromatic hydrocarbons in LCO will undergo hydrosaturation, ring-opening and cracking reactions to transform into bicyclic aromatic hydrocarbons. Similarly, tricyclic cycloalkanes will also be converted into bicyclic cycloalkanes through ring-opening and cracking reactions. However, accurately distinguishing whether these bicyclic aromatic hydrocarbons and cycloalkanes originate from LCO feedstock or reaction generation remains challenging. Since they are intermediate products, a simplified calculation method is adopted in Equation (2). This method does not account for the ring-opening rate of tricyclic aromatic hydrocarbons and cycloalkanes to bicyclic aromatic hydrocarbons and cycloalkanes, which may lead to an underestimation of the ring-opening rate.
RESULTS AND DISCUSSION
Aromatic distributions and typical molecular structures in LCO and HDT-LCO
The hydrocarbon compositions of LCO and HDT-LCO are presented in Table 1, showing that the total aromatic content in LCO is 89.0%. Among these aromatic hydrocarbons, the bicyclic aromatic hydrocarbons account for 68.4% of the total, with a content of 60.9%. Bicyclic aromatic hydrocarbons mainly include naphthalene, acenaphthene, fluorene, and their alkyl-substituted derivatives according to molecular structure, among which naphthalene and alkyl-substituted naphthalenes are the most abundant. In contrast to LCO, the remaining PAHs including bicyclic and tricyclic aromatic hydrocarbons in HDT-LCO show a significant decrease, while the levels of monocyclic aromatic hydrocarbons, cycloalkanes, and alkanes exhibit a notable increase. Among these changes, the most substantial rise is observed in the content of monocyclic aromatic hydrocarbons, particularly those tetralin or indane-like structures. As previously mentioned, certain PAHs present in LCO undergo partial hydro-saturation reaction during the hydrotreating process and are converted into tetralin-like monocyclic aromatic hydrocarbons, which serve as primary reactants for subsequent hydrocracking reactions.
Hydrocarbon compositions of LCO and HDT-LCO
| Mass fraction of hydrocarbons/% | LCO | HDT-LCO |
| Paraffins | 7.8 | 9.9 |
| Mono-cycloalkanes | 0.9 | 0.9 |
| Bicyclic cycloalkanes | 1.8 | 4.7 |
| Tricyclic cycloalkanes | 0.5 | 1.6 |
| Total cycloalkanes | 3.2 | 7.2 |
| Total aromatic hydrocarbons | 89.0 | 82.9 |
| Alkylbenzenes | 9.7 | 10.4 |
| Tetralins or indans | 6.8 | 31.7 |
| Indenes and/or CnH2n-10 | 1.5 | 8.4 |
| Total monocyclic aromatic hydrocarbons | 18.0 | 50.5 |
| Naphthalenes | 38.4 | 10.8 |
| Acenaphthenes and/or CnH2n-14 | 11.8 | 10.8 |
| Fluorenes and/or CnH2n-16 | 10.7 | 7.8 |
| Total bicyclic aromatic hydrocarbons | 60.9 | 29.4 |
| Tricyclic aromatic hydrocarbons | 10.1 | 3.0 |
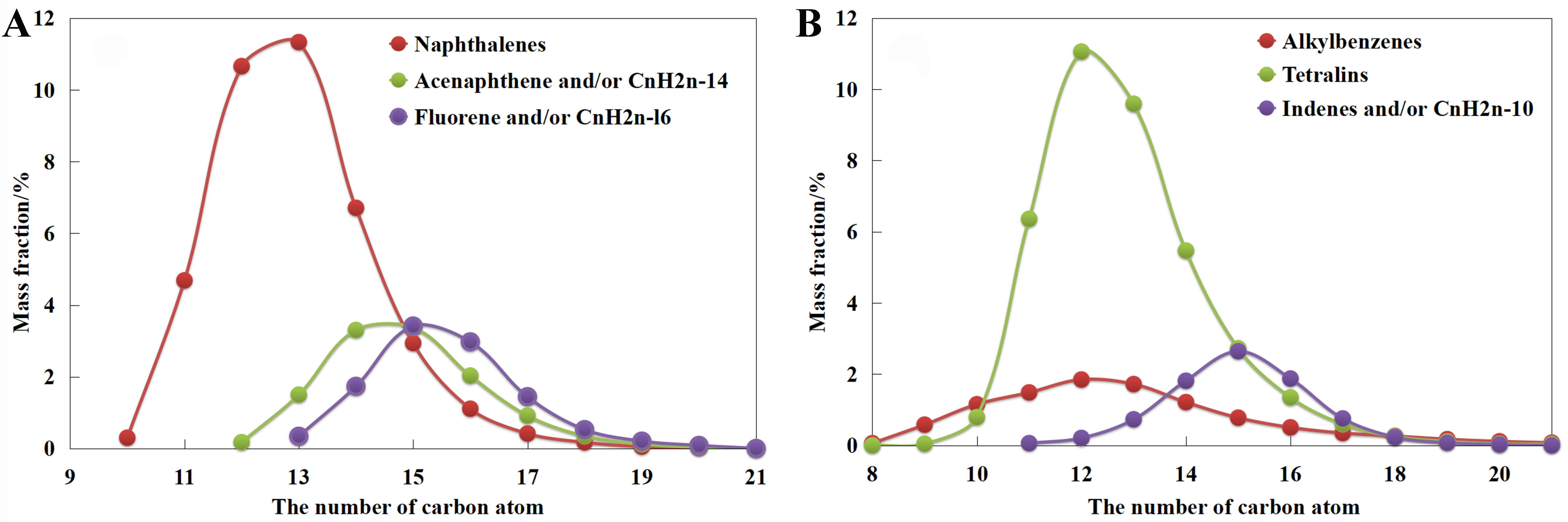
To obtain the molecular structure, we conducted the carbon number distributions of bicyclic aromatic hydrocarbons in LCO and monocyclic aromatic hydrocarbons in corresponding HDT-LCO. As illustrated in [Figure 2], alkylated naphthalenes in LCO predominantly exhibit a carbon number ranging from 11 to 14, while the alkylated acenaphthenes and fluorenes mainly fall within the range of 14 to 16. This suggests that the bicyclic aromatic hydrocarbons in LCO are predominantly alkyl-substituted with 1-4 side chain carbon atoms. Similarly, monocyclic aromatic hydrocarbons in HDT-LCO mainly consist of tetralin-like compounds with a carbon number ranging from 11 to 14. This is attributed to the fact that polycyclic aromatic compounds primarily undergo hydro-saturation reactions during the hydrotreating process, while the dealkalation and cracking of alkyl side chains are almost negligible.
Figure 2. (A) Carbon number distribution of bicyclic aromatic hydrocarbons in LCO and (B) monocyclic aromatic hydrocarbons in HDT-LCO. LCO: Light cycle oil; HDT-LCO: hydrotreated LCO.
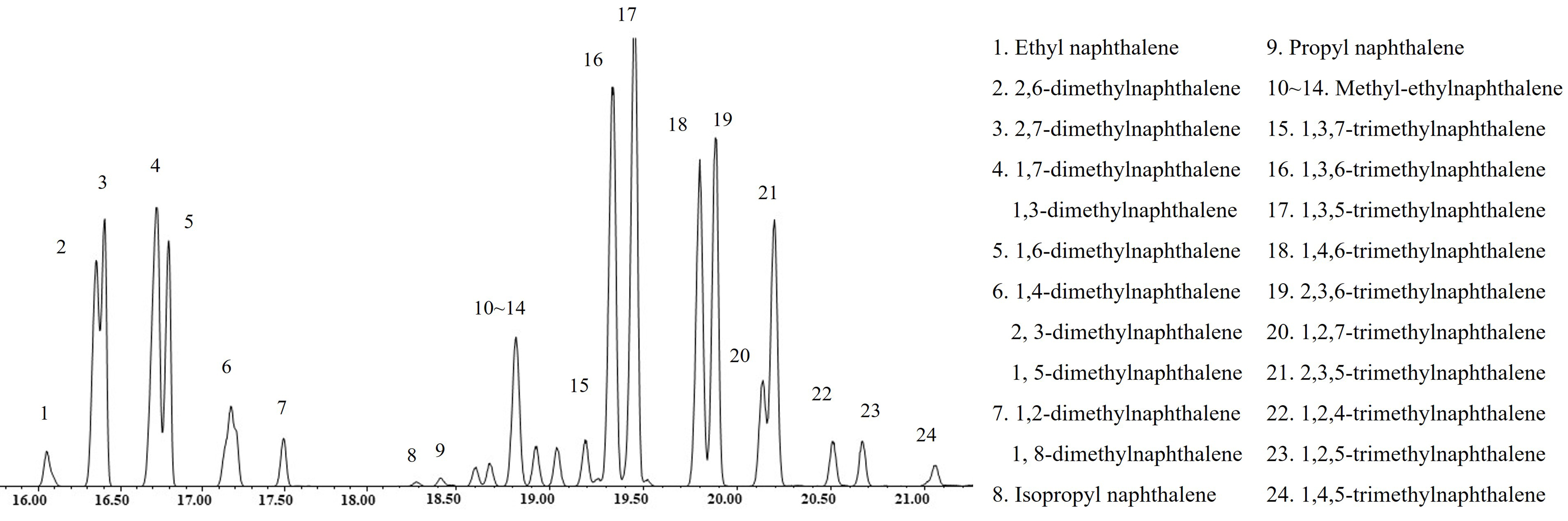
GC-MS is utilized to further identify the predominant bicyclic aromatic hydrocarbons in LCO for calculating their molecule sizes, with a specific focus on alkyl-substituted naphthalenes with carbon atom numbers of 12 and 13. As illustrated in Figure 3, alkyl-substituted naphthalenes with C12 predominantly consist of various isomers including dimethylnaphthalene, along with a minor presence of ethylnaphthalene. Similarly, alkyl-substituted naphthalenes with C13 primarily consist of diverse isomers such as trimethylnaphthalene, accompanied by a small quantity of methylethyl naphthalene and propylnaphthalene. These findings indicate that the PAHs in LCO mainly comprise polysubstituted alkyl-aromatic compounds featuring short side chains, which undergo transformation into the corresponding tetralin-like monocyclic aromatic hydrocarbons in HDT-LCO during the hydrotreating process.
Table 2 presents the three-dimensional dimensions of the predominant alkyl-naphthalenes and corresponding alkyltetralins in C12 and C13. It is evident that these molecules are significantly larger than the micropores of HY zeolite (0.74 nm × 0.74 nm), rendering them unable to penetrate the pores of the zeolite.
The three-dimensional dimensions of typical C12 and C13 naphthalenes and tetralins
| Molecule | Structure | a × b × c / nm × nm × nm |
| 2,7-dimethylnaphthalene | 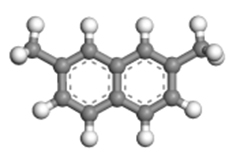 | 1.10 × 0.72 × 0.40 |
| 1,7-dimethylnaphthalene | 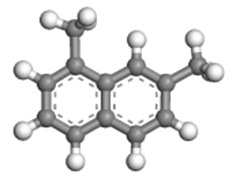 | 1.01 × 0.83 × 0.41 |
| 1,3,6-trimethylnaphthalene | 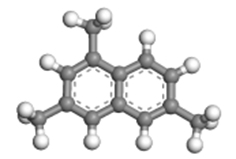 | 1.09 × 0.85 × 0.40 |
| 1,3,5-trimethylnaphthalene | 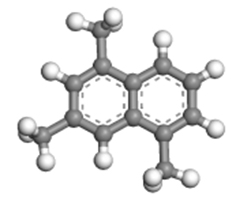 | 0.99 × 0.87 × 0.40 |
| 2,7-dimethyltetralin | 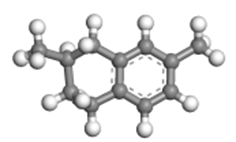 | 1.14 × 0.72 × 0.53 |
| 1,7-dimethyltetralin | 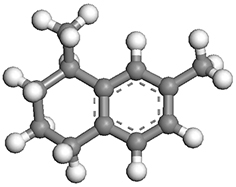 | 1.03 × 0.83 × 0.53 |
| 1,3,6-trimethyltetralin | 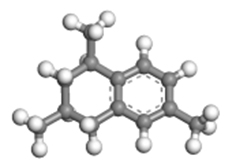 | 1.12 × 0.85 × 0.52 |
| 1,3,5-trimethyltetralin | 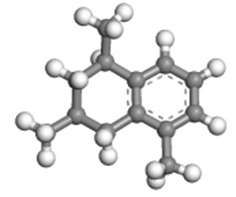 | 1.02 × 0.89 × 0.53 |
Quantitative analysis of physicochemical property and BAS accessibility in HY zeolites
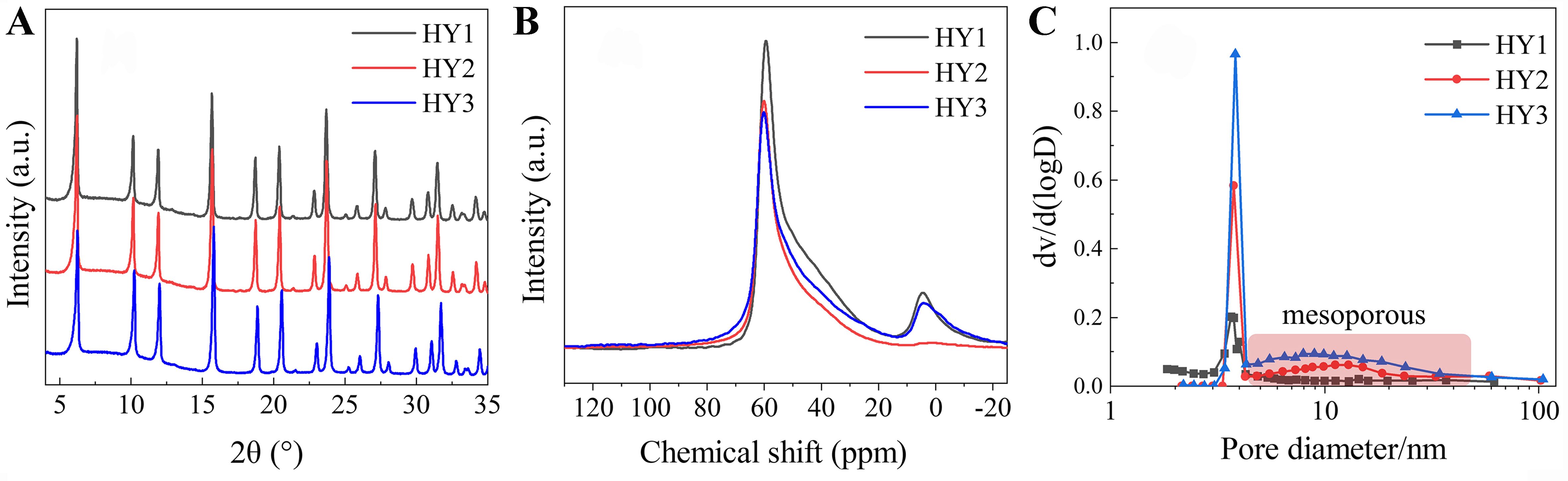
XRD characterization of the three HY zeolite samples was conducted and the results were depicted in Figure 4A. All the samples display typical diffraction peaks of the FAU topology structure zeolite. No significant additional peaks are observable.
Figure 4. (A) XRD pattern, (B) 27Al MAS NMR spectra, (C) pore size distribution of HY zeolites. XRD: X-ray diffraction; MAS NMR: magic angle spinning nuclear magnetic resonance.
The aluminum coordination states on the three HY zeolites are illustrated in Figure 4B. The peak with a chemical shift (δ) at approximately 60 ppm corresponds to the tetrahedrally coordinated framework aluminum (FAl), while the peak with δ at around 0 ppm belongs to the octahedrally coordinated Extra-FAl (EFAl). The assignment of the peaks with chemical shifts between 30 and 50 ppm is still under debate, but they are generally considered as distorted tetrahedral or penta-coordinated EFAl. As depicted in Figure 4B, all the HY zeolites predominantly contain FAl, with a minor presence of EFAl. Among them, HY2 exhibits the lowest content of EFAl.
Table 3 and Supplementary Figure 1 show the textural properties of the HY zeolites. The specific surface areas of these zeolites range from 651 to 700 m2/g, and the pore volumes vary from 0.355 to 0.400 cm3/g. The mesoporous specific surface area and mesoporous pore volume increase gradually from HY1 to HY3. It can be seen from Figure 4C that there is a gradual increase in the proportion of mesopores (primarily 10-40 nm) in zeolites from HY1 to HY3, which could influence the accessibility of acidic centers.
Texture properties of HY zeolites
| Zeolite | Specific surface area/(m2/g) | Pore volume/(cm3/g) | ||||
| SBET | SMicro | Smeso | Vpore | Vmicro | Vmeso | |
| HY1 | 700 | 670 | 31 | 0.355 | 0.310 | 0.045 |
| HY2 | 641 | 608 | 33 | 0.365 | 0.287 | 0.078 |
| HY3 | 651 | 619 | 63 | 0.460 | 0.290 | 0.170 |
FTIR is usually employed to qualitatively characterize the acidic sites of the zeolites. Many basic molecules, such as small alkaline probe molecules (ammonia and Py) and large probe molecules (multi-alkylated Py or other organic amine molecules), can be used to characterize the acidity of samples. The selection of different alkaline probe molecules can provide diverse information on acidity. Therefore, it is desirable and highly challenging to choose the appropriate alkaline probe molecule based on the chemical properties and molecular size of reactants.
Three alkaline probe molecules with different molecular sizes and chemical properties were selected to assess the accessibility of acid sites in HY zeolites, aiming to investigate their impacts on the performance of LCO hydrocracking for light aromatic hydrocarbon production. The adsorption energies on the external surface BAS were calculated by DFT (B3LYP/6-31 + G**). Table 4 shows the total acid amount and external surface Brønsted acid amount of the three HY zeolites. Considering its molecular size (~0.57 nm), Py can approach almost all the acidic sites of the zeolites. Thus, the acid amount measured by Py is defined as the total acid amount. Additionally, 2,4,6-TTBPy (~1.1 nm) and THA (~1.4 nm) were chosen as probe molecules, resembling the aromatics in LCO and HDT-LCO [Table 2], which can only approach the acidic sites on the external surface of the zeolites due to their large molecular size. The acid amount measured by these molecules is defined as external surface acidity.
Acid properties of HY zeolites
| Zeolites | Total acid amount mL NH3/g | Total acid amount on BAS by Py/(μmol/g) | External surface acid amount of BAS by 2,4,6-TTBPy/(μmol/g) | External surface acid amount of BAS by THA/(μmol/g) |
| HY1 | 36.77 | 101 | 27 | 40 |
| HY2 | 36.58 | 109 | 5 | 48 |
| HY3 | 25.97 | 92 | 3 | 87 |
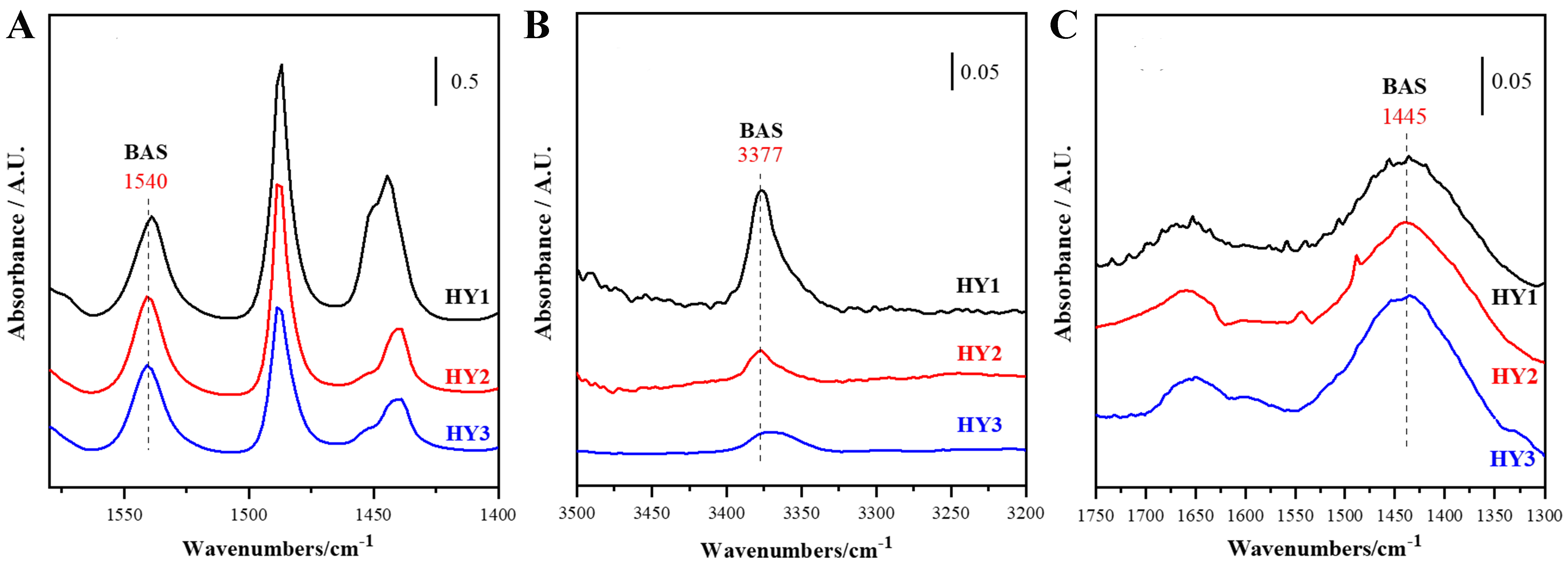
As shown in Figure 5, 1,540 cm-1 of Py adsorption, 3,377 cm-1 of 2,4,6-TTBPy adsorption, and 1,450 cm-1 of THA adsorption on zeolites are attributed to the presence of BAS. Table 4 shows that the total Brønsted acid amount measured by Py gradually decreases from HY1 to HY3, while the external surface Brønsted acid amount measured by THA shows a gradual increase. The external surface Brønsted acid amount measured by 2,4,6-TTBPy is exceedingly low and also exhibits a gradual decrease. The three alkaline probe molecules provide totally different information on acidity of these HY zeolites.
Figure 5. Acid amount on BAS measured by (A) Py and external surface BAS measured by (B) 2,4,6-TTBPy and (C) THA of the three HY zeolites. BAS: Brønsted acid sites; Py: pyridine; 2,4,6-TTBPy: 2,4,6-tri-tert-butylpyridine; THA: trihexylamine.
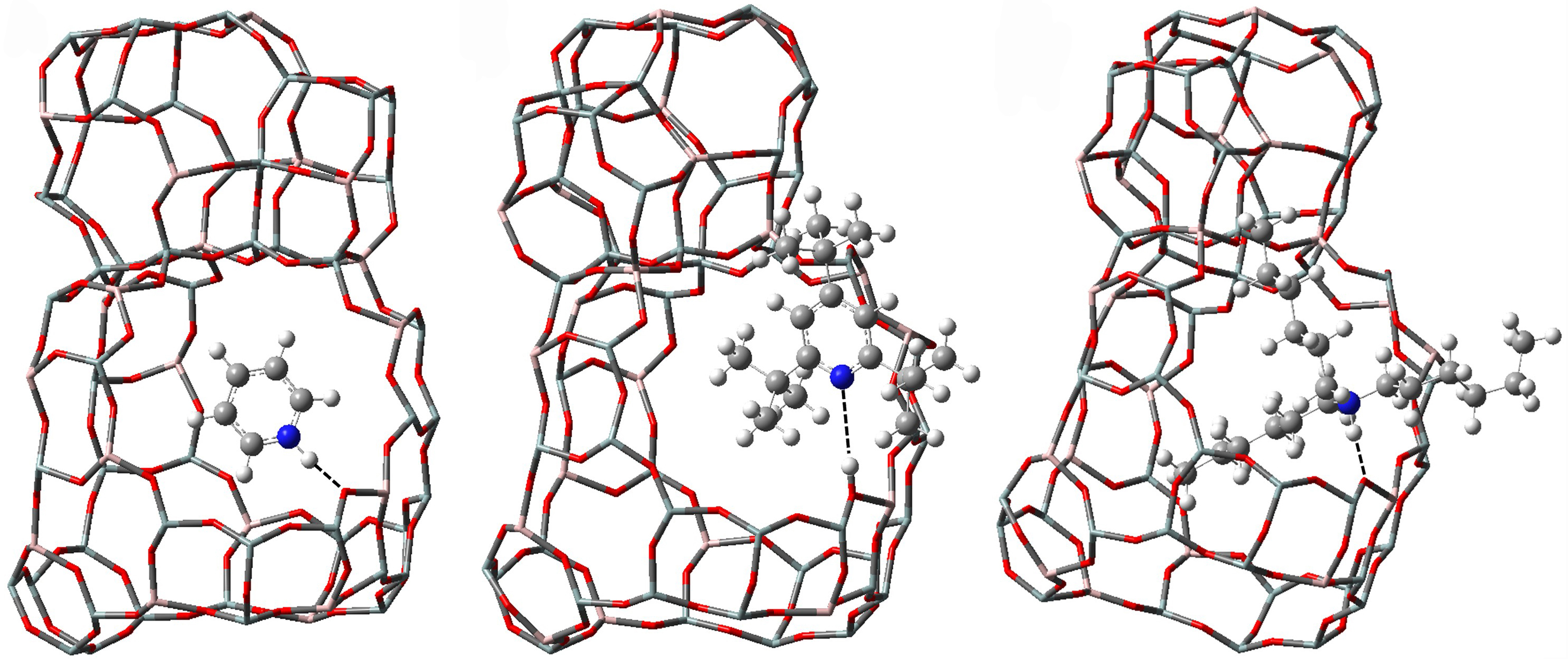
The calculation results from Figure 6 and Table 5 indicate that Py exhibits strong ion adsorption with BAS. Among the probe molecules, THA possesses the largest molecular size, which hinders its entry into zeolite channels. However, due to its quasi-planar structure, it exhibits relatively small steric hindrance with the zeolite framework, resulting in relatively strong ion adsorption on the surface BAS. In contrast, 2,4,6-TTBPy has a smaller molecular size with THA, but its increased steric hindrance between the three tert-butyl branches and the zeolite framework prevents nitrogen on the Py ring from interacting with the BAS and leads to the reduced adsorption energy. Therefore, the Brønsted acid amount measured by 2,4,6-TTBPy would be underestimated.
Figure 6. Structures of Py, 2,4,6-TTBPy and THA adsorbed on BAS of HY zeolite. Py: Pyridine; 2,4,6-TTBPy: 2,4,6-tri-tert-butylpyridine; THA: trihexylamine; BAS: Brønsted acid sites.
Adsorption energy and some key bond lengths of Py, 2,4,6-TTBPy and THA adsorbed on HY zeolites
| Probe molecule | ∆Eads/kcal/mol | Bond length/Å | |
| N–H | H–O | ||
| Py | -36.14 | 1.06 | 1.64 |
| 2,4,6-TTBPy | -8.64 | 3.06 | 0.98 |
| THA | -33.89 | 1.06 | 1.71 |
LCO hydrocracking process and the effects of BAS accessibility on catalytic performance
As stated in Figure 1, monocyclic aromatic hydrocarbons in HDT-LCO undergo isomerization, ring-opening, and cracking reactions on the hydrocracking catalysts to yield desirable BTX products. Additionally, side reactions such as over-cracking, excessive hydrosaturation, and polymerization coking are also facilitated. Based on their distinct boiling points, the products are classified into C1-C4 gases, naphtha ranging from C5 to 478 K, and unconverted diesel above 478 K. The naphtha products are further divided into C5-C12 paraffins, C5-C12 cycloalkanes, and C6-C12 aromatic hydrocarbons according to carbon number and molecular structure.
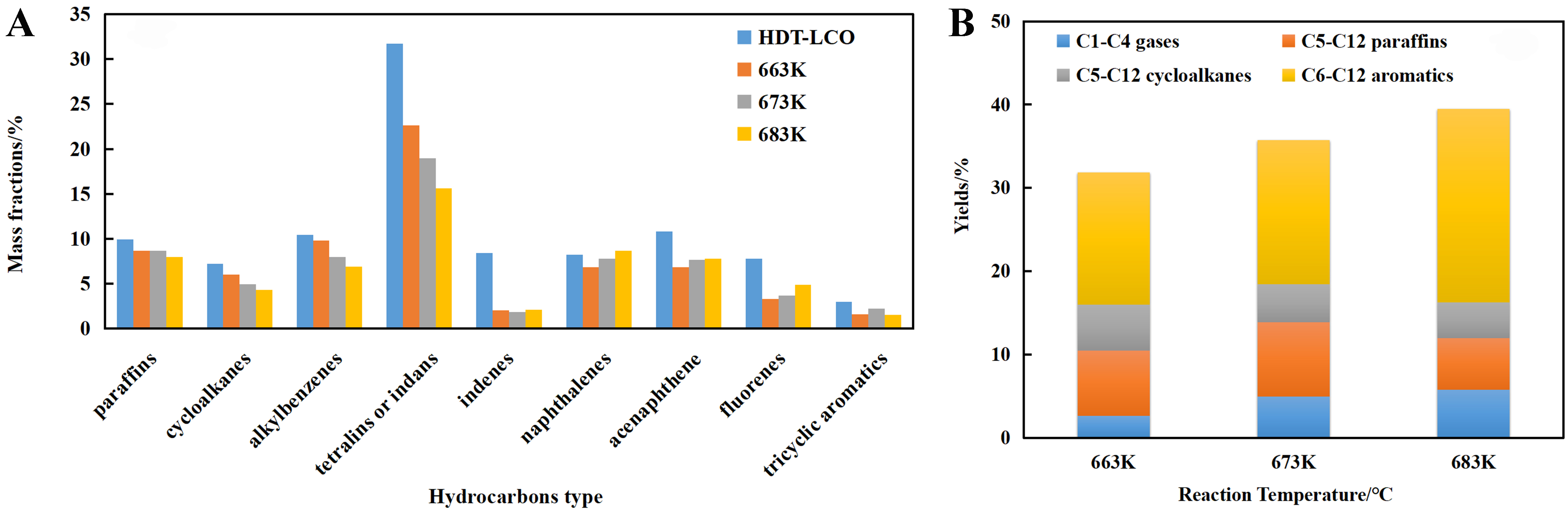
A comparative analysis was conducted to investigate the hydrocracking process of HDT-LCO, focusing on changes of hydrocarbons in unconverted diesel and distributions of product (Catalyst 1, C1). As illustrated in Figure 7A, there is a significant reduction in levels of monocyclic aromatic hydrocarbons, such as alkylbenzenes, indanes or tetralins, and indenes compared to the HDT-LCO feedstock. Additionally, an increase in reaction temperature leads to reduced paraffin contents along with cycloalkanes, alkylbenzenes and indanes or tetralins. However, the contents of bicyclic and tricyclic aromatic hydrocarbons initially decrease before subsequently rising. This phenomenon may be attributed to thermodynamic equilibrium limitations on hydrosaturation reaction for aromatic hydrocarbons at elevated temperatures.
Figure 7. (A) Changes of hydrocarbons in unconverted diesel and (B) distributions of product on C1 catalyst.
Under the experimental conditions [Figure 7B], C6-C12 aromatic hydrocarbons are identified as the main products. With rising reaction temperature, there is a continuous increase in the yield of C6-C12 aromatic hydrocarbons, a gradual increase in the yield of C1-C4 gaseous products, and a slight decrease in the yield of C6-C12 cycloalkanes. The yield of C6-C12 paraffins initially increases before exhibiting a decreasing trend.
These observations indicate that, within the hydrocracking reaction zone, minimal amounts of cycloalkanes and paraffins in HDT-LCO undergo cracking reactions, and ring-opening reactions of tetralin-like monocyclic aromatic hydrocarbons and cracking reaction of corresponding ring-opening products predominantly occur, leading to the formation of C6-C12 aromatic hydrocarbons along with minor amount of gaseous and light saturated hydrocarbons.
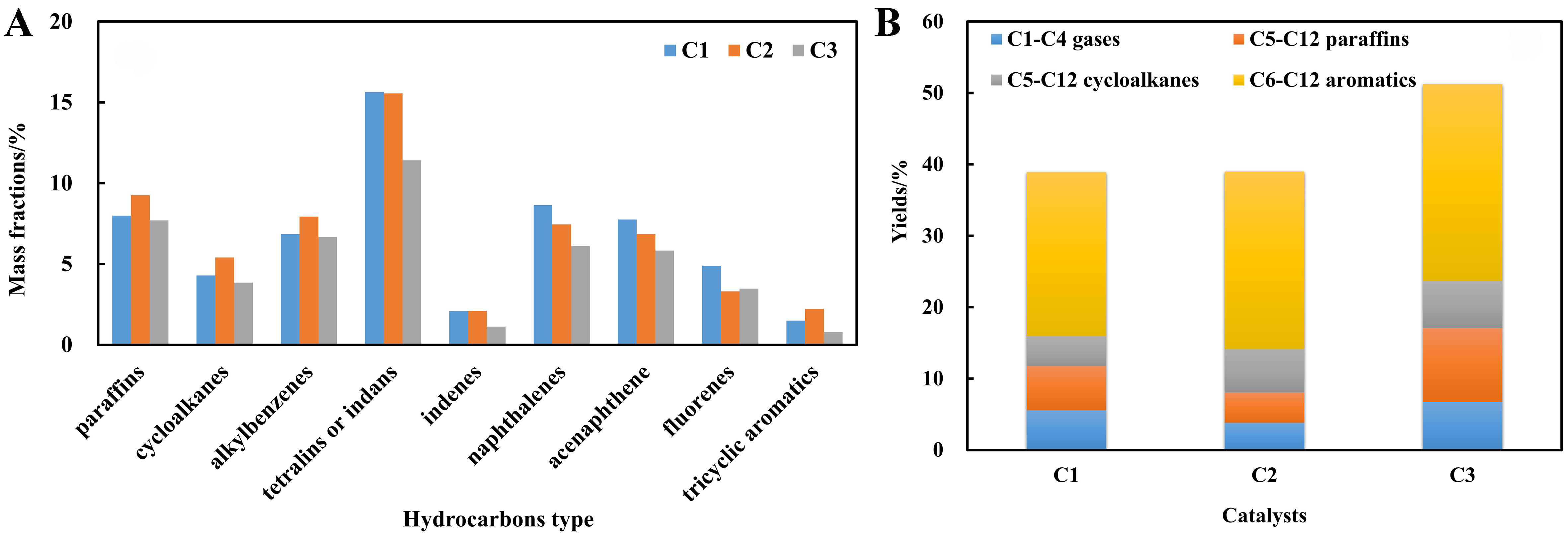
A comprehensive comparison of product distribution over the three catalysts is presented in Table 6, demonstrating a gradual increase in the conversion of HDT-LCO from C1 to C3. Moreover, the conversion was enhanced with rising reaction temperature. The hydrocarbon changes in unconverted diesel and product distribution at 683 K on the three catalysts are further illustrated in Figure 8. As shown in Figure 8A, the aromatic hydrocarbons in unconverted diesel on C3 catalyst, including monocyclic, bicyclic, and tricyclic compounds, are the lowest, followed by C2 and C1. Consequently, as depicted in Figure 8B, the yields of C1-C4 gases, C5-C12 alkanes, C5-C12 cycloalkanes and C6-C12 aromatic hydrocarbons produced by the C3 catalyst are the highest among all catalysts examined. Additionally, C2 catalyst exhibits slightly higher
Figure 8. Influences of catalysts on (A) changes of hydrocarbons in unconverted diesel and (B) distributions of product.
Variation of product distribution with temperature on three catalysts
| Yields/% | C1 | C2 | C3 | ||||||
| 663 K | 673 K | 683 K | 663 K | 673 K | 683 K | 663 K | 673 K | 683 K | |
| C1-C4 gases | 2.61 | 4.76 | 5.56 | 3.18 | 3.24 | 3.82 | 3.55 | 5.09 | 6.76 |
| Naphtha | 29.15 | 30.37 | 33.37 | 33.64 | 34.09 | 35.18 | 40.61 | 43.81 | 44.46 |
| Unconverted diesel | 68.24 | 64.86 | 61.07 | 63.17 | 62.67 | 61.01 | 55.85 | 51.09 | 48.77 |
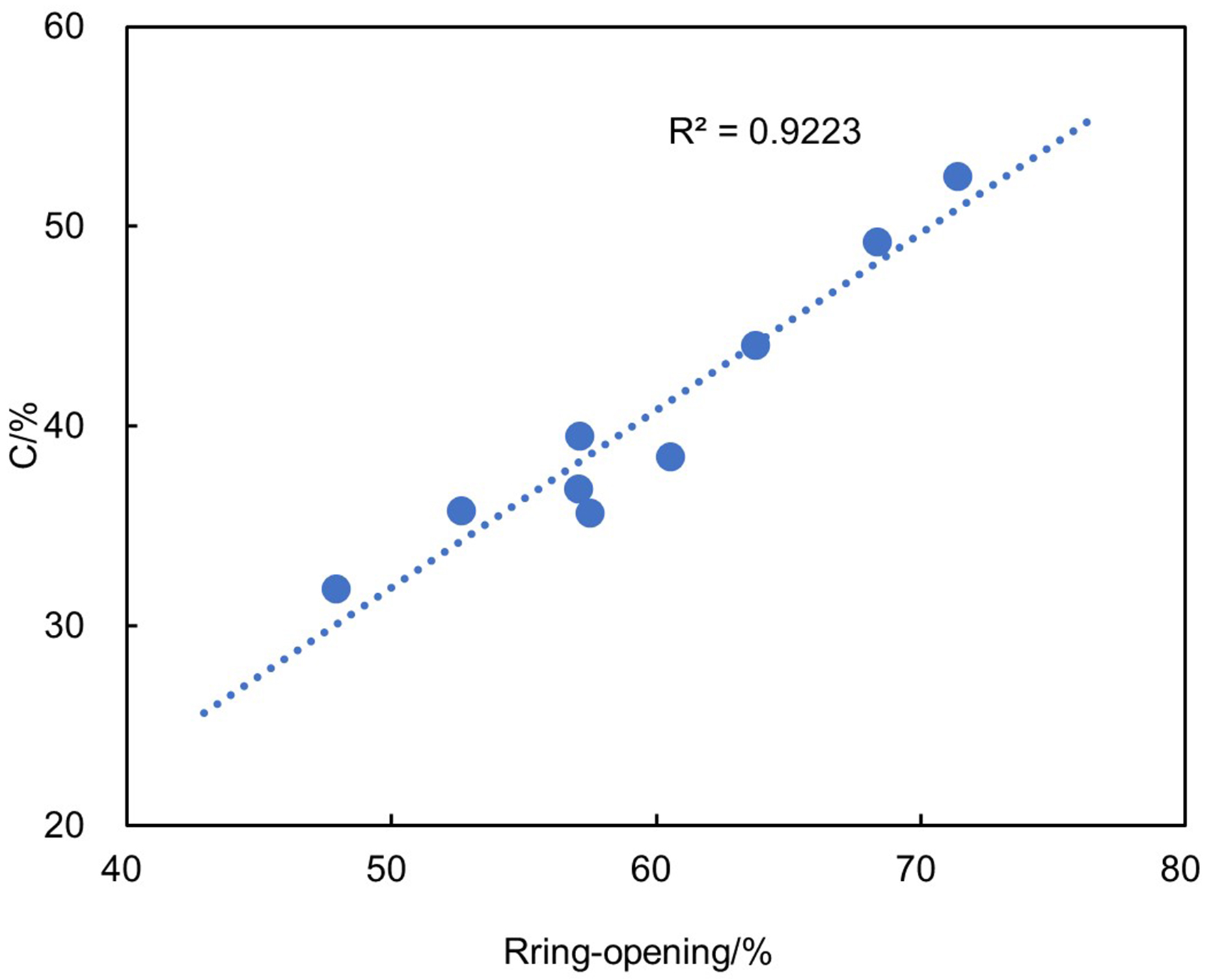
Although the LCO conversions and product yields differ among the three catalysts, there is a notable increase in the LCO conversion as the ring-opening rate of PAHs and polycyclic cycloalkanes in LCO increases [Figure 9]. A strong linear correlation is evident, further affirming that the ring-opening reaction plays a crucial role in determining the reaction activity during LCO hydrocracking processing.
Figure 9. Relationship between the ring-opening rate of PAHs and LCO conversion. PAHs: Polycyclic aromatic hydrocarbons; LCO: light cycle oil.
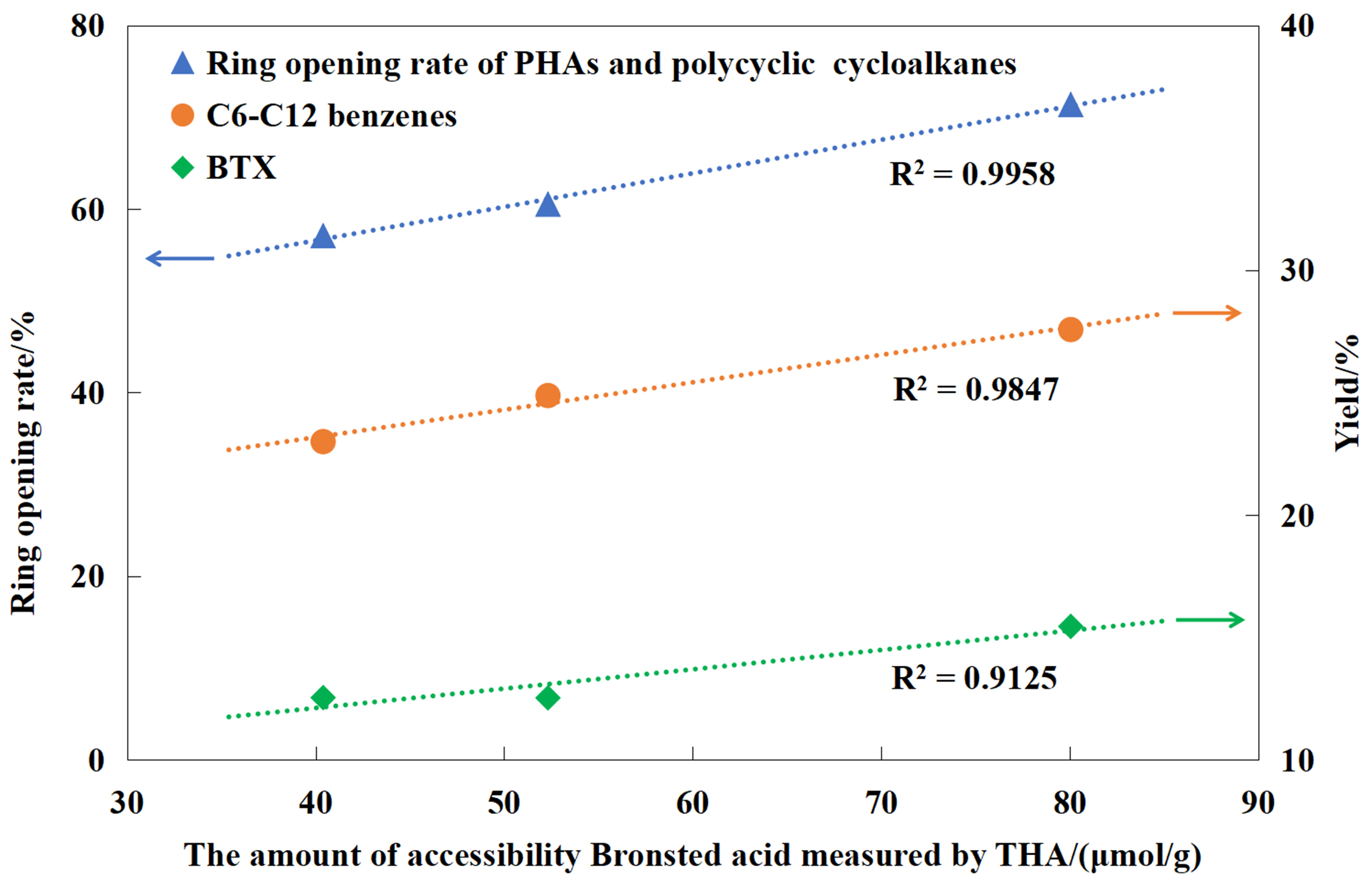
Figure 10 revealed a strong positive correlation between the amount of accessibility Brønsted acid on HY zeolites determined by THA and the ring-opening rate for PAHs and polycyclic cycloalkanes, as well as the yield of C6-C12 benzenes and BTX. As a heterogeneous catalytic process, the initial and key step of the ring-opening reaction involves diffusion and adsorption of reactant molecules onto acidic sites. While both THA and 2,4,6-TTBPy are unable to enter HY zeolite micropores, it is worth noting that THA molecules exhibit smaller steric hindrance compared to 2,4,6-TTBPy. This makes it more similar to the adsorption behavior of aromatics in LCO and HDT-LCO (the C–H and O–H bond strengths of 2,7-dimethylteralin adsorbed on HY zeolites are 2.08 and 0.99 Å, respectively). Therefore, THA is better suited for characterizing the accessibility of aromatics in LCO and HDT-LCO to the acidic sites over HY zeolite hydrocracking catalysts.
CONCLUSIONS
The hydrocracking of LCO to produce light aromatic hydrocarbons is an effective approach for addressing excess diesel production capacity and facilitating the high-quality transformation of refineries into chemicals. LCO primarily consists of bicyclic aromatics with multiple, short side chains ranging from C1 to C4, such as dimethylnaphthalene and trimethylnaphthalene. These compounds are transformed into monocyclic aromatic hydrocarbons such as alkyltetralins with minimal disruption to the side chain after hydrotreating. During the subsequent hydrocracking process, the alkyltetralins undergo ring opening and cracking tandem reaction, yielding C1-C4 gases, C5-C12 paraffins, C5-C12 cycloalkanes and C6-C12 light aromatics, with the latter being the main products. There is a strong positive correlation between the conversion and the ring-opening rate of PAHs and polycyclic cycloalkanes in LCO.
The dimensions of typical alkyl naphthalenes in LCO and the corresponding alkyl tetralins in HDT-LCO are larger than those of micropores over HY zeolites. This makes it difficult for them to effectively enter the pores for adsorption onto BAS or subsequent reaction. Therefore, three hierarchical porous HY zeolites possessing comparable Si/Al molar atomic ratios and BAS were selected to investigate acid accessibility and their effects on LCO hydrocracking. The analysis of THA adsorption in FTIR indicates that the proportion and quantity of the external surface BAS on HY zeolites increase in the order of HY1 < HY2 < HY3, which is in good consistency with product distribution, particularly evident through the increasing trend of ring-opening rate of PAHs and polycyclic cycloalkanes, yields of C6-C12 light aromatic hydrocarbons and BTX. By employing THA in combination with FTIR, a strong correlation has been established between acid accessibility and the performance of LCO hydrocracking on HY zeolite catalysts. These findings can provide fundamental information for the development of high-performance hydrocracking catalysts and technologies.
DECLARATIONS
Authors’ contributions
Carried out the preparation, physical property characterization and tests of the catalysts, and prepared the draft manuscript: Yang, P.
Performed FTIR characterization of the catalysts and prepared the draft manuscript: Yan, S.
Performed FTIR characterization of the catalysts: Guo, L.
Performed the DFT calculation: He, N.
Corrected the manuscript: Xiong, G.
Performed GC-MS characterization of LCO and HDT-LCO: Wang, N.
Planned the study: Liu, J.; Li, M.; Nie, H.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the involvement of trade secrets, but are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This research is supported by the National Key Research and Development Program of China (2022YFA1504402), the National Energy R&D Center of Petroleum Refining Technology (RIPP, SINOPEC) and “the Fundamental Research Funds for the Central Universities” (DUT22LAB601, etc.).
Conflicts of interest
Yang, P.; Nie, H.; Wang, N.; and Li, M. are affiliated with SINOPEC Research Institute of Petroleum Processing Co., Ltd, while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Ramteke, A. V.; Bhatia, D.; Pant, K. Conversion of light cycle oil to benzene and alkylated monoaromatics over monometallic and bimetallic CoMo catalysts in the presence of hydrogen donor. Fuel 2023, 342, 127737.
2. Corma, A.; González-Alfaro, V.; Orchillés, A. Decalin and tetralin as probe molecules for cracking and hydrotreating the light cycle oil. J. Catal. 2001, 200, 34-44.
3. Peng, C.; Huang, X.; Duan, X.; et al. Direct production of high octane gasoline and ULSD blend stocks by LCO hydrocracking. Catal. Today. 2016, 271, 149-53.
4. Zhang, L.; Tang, X.; Li, J.; Yang, J.; Dai, G. Extraction and separation of polycyclic aromatic hydrocarbons from catalytic cracking diesel. J. Chem. Eng. Data. 2023, 68, 393-404.
5. Miao, P.; Miao, J.; Guo, Y.; Lin, C.; Zhu, X.; Li, C. Efficient conversion of light cycle oil into gasoline with a combined hydrogenation and catalytic cracking process: effect of pre-distillation. Energy. Fuels. 2020, 34, 12505-16.
6. Xin, L.; Liu, X.; Chen, X.; Feng, X.; Liu, Y.; Yang, C. Efficient conversion of light cycle oil into high-octane-number gasoline and light olefins over a mesoporous ZSM-5 catalyst. Energy. Fuels. 2017, 31, 6968-76.
7. Laredo, G. C.; Pérez-Romo, P.; Escobar, J.; Garcia-Gutierrez, J. L.; Vega-Merino, P. M. Light cycle oil upgrading to benzene, toluene, and xylenes by hydrocracking: studies using model mixtures. Ind. Eng. Chem. Res. 2017, 56, 10939-48.
8. Oh, Y.; Noh, H.; Park, H.; Han, H.; Nguyen, T.; Lee, J. K. Molecular-size selective hydroconversion of FCC light cycle oil into petrochemical light aromatic hydrocarbons. Catal. Today. 2020, 352, 329-36.
9. Cao, Z.; Zhang, X.; Xu, C.; et al. Selective hydrocracking of light cycle oil into high-octane gasoline over bi-functional catalysts. J. Energy. Chem. 2021, 52, 41-50.
10. Oh, Y.; Shin, J.; Noh, H.; et al. Selective hydrotreating and hydrocracking of FCC light cycle oil into high-value light aromatic hydrocarbons. Appl. Catal. A. Gen. 2019, 577, 86-98.
11. Escalona, G.; Rai, A.; Betancourt, P.; Sinha, A. K. Selective poly-aromatics saturation and ring opening during hydroprocessing of light cycle oil over sulfided Ni-Mo/SiO2-Al2O3 catalyst. Fuel 2018, 219, 270-8.
12. Chen, F.; Zhang, G.; Weng, X.; et al. High value utilization of inferior diesel for BTX production: mechanisms, catalysts, conditions and challenges. Appl. Catal. A. Gen. 2021, 616, 118095.
13. Qi, L.; Peng, C.; Cheng, Z.; Zhou, Z. Structure-performance relationship of NiMo/Al2O3-HY catalysts in selective hydrocracking of poly-aromatics to mono-aromatics. Chem. Eng. Sci. 2022, 263, 118121.
14. Peng, C.; Zhou, Z.; Cheng, Z.; Fang, X. Upgrading of light cycle oil to high-octane gasoline through selective hydrocracking over non-noble metal bifunctional catalysts. Energy. Fuels. 2019, 33, 1090-7.
15. Du, H.; Fairbridge, C.; Yang, H.; Ring, Z. The chemistry of selective ring-opening catalysts. Appl. Catal. A. Gen. 2005, 294, 1-21.
16. Santikunaporn, M.; Herrera, J.; Jongpatiwut, S.; Resasco, D.; Alvarez, W.; Sughrue, E. Ring opening of decalin and tetralin on HY and Pt/HY zeolite catalysts. J. Catal. 2004, 228, 100-13.
17. Lee, J.; Choi, Y.; Shin, J.; Lee, J. K. Selective hydrocracking of tetralin for light aromatic hydrocarbons. Catal. Today. 2016, 265, 144-53.
18. Arribas, M. A.; Martínez, A.; Sastre, G. Simultaneous hydrogenation and ring opening of aromatics for diesel upgrading on Pt/zeolite catalysts. The influence of zeolite pore topology and reactant on catalyst performance. Stud. Surf. Sci. Catal. 2002, 142, 1015-22.
19. Arribas, M.; Corma, A.; Díaz-cabañas, M.; Martínez, A. Hydrogenation and ring opening of Tetralin over bifunctional catalysts based on the new ITQ-21 zeolite. Appl. Catal. A. Gen. 2004, 273, 277-86.
20. Qi, J.; Guo, Y.; Jia, H.; et al. Unpredictable properties of industrial HY zeolite for tetralin hydrocracking. Fuel. Proc. Technol. 2023, 240, 107586.
21. Sharma, P.; Iguchi, Y.; Sekine, Y.; Kikuchi, E.; Matsukata, M. 42 Hydroisomerization of tetralin on zeolite beta: influence of crystal size. Stud. Surf. Sci. Catal. 2003, 145, 219-22.
22. Nesterenko, N.; Thibault-Starzyk, F.; Montouillout, V.; et al. Accessibility of the acid sites in dealuminated small-port mordenites studied by FTIR of co-adsorbed alkylpyridines and CO. Micropor. Mesopor. Mat. 2004, 71, 157-66.
23. Mlekodaj, K.; Tarach, K.; Datka, J.; Góra-Marek, K.; Makowski, W. Porosity and accessibility of acid sites in desilicated ZSM-5 zeolites studied using adsorption of probe molecules. Micropor. Mesopor. Mat. 2014, 183, 54-61.
24. Thibault-Starzyk, F.; Stan, I.; Abelló, S.; et al. Quantification of enhanced acid site accessibility in hierarchical zeolites - The accessibility index. J. Catal. 2009, 264, 11-4.
25. Zhang, L.; Hu, Q.; Qin, Y.; et al. Optimizing the accessibility of zeolite Y on FCC catalyst to improve heavy oil conversion capacity. Micropor. Mesopor. Mat. 2023, 359, 112627.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Author Biographies























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].