A novel hydrogen-bonded mesoporous framework nanomaterials
Keywords
Based on the molecular-level assembly, hierarchical mesoporous nanostructures with architectural complexity, pore tunability, and special functionalities have gained widespread attention in materials science and engineering[1,2]. Over the past decades, mesoporous nanostructures of different sizes, structures, or properties have been reported, and these different nanostructured materials have demonstrated excellent properties in various fields, involving optics, electronics, and catalysis[3-5]. To date, as researchers continue to discover and explore, this “molecular-level” oligomer ultra-small structure is no longer sufficient for material needs. In order to expand the “family” members of the appealing porous materials, the construction of hierarchical mesoporous frameworks via the modular self-assembly of subnanoscale-level, even nanoscale-level building units has turned a hot topic[6]. Similar to the prominent porous materials of covalent-organic frameworks (COFs), metal-organic frameworks (MOFs), and hydrogen-bonded organic frameworks (HOFs), the multilevel mesoporous nanocluster frameworks are of great potential in multiple applications[7-9]. So far, precisely regulating the interactions between the pore-forming agents and the nanocluster units and further controlling the assembly behavior to form the porous frameworks remain a great challenge. It is difficult to form these porous frameworks when the binding force between the “glue-like” linkers (e.g., amphiphilic micelles) and the cluster units is either too strong or weak[10]. If the binding force is too strong (such as ionic bonds or covalent bonds), it may lead to a collapse of porous architecture. If it is too weak (e.g., van der Waals force or hydrophilic-hydrophobic interaction), the building units of clusters typically cannot be assembled or anchored on the surface of the pore-forming agents of the micelles.
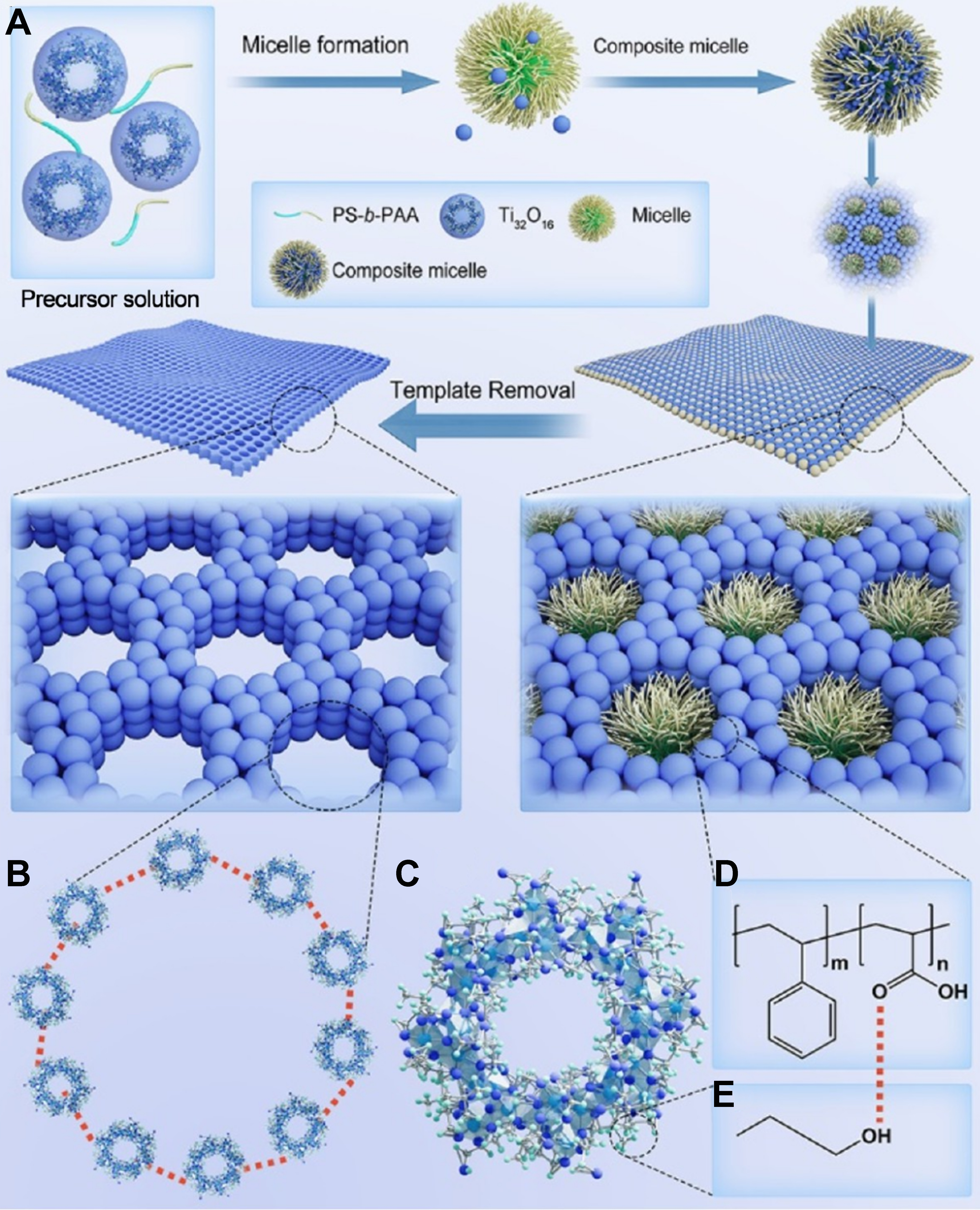
In order to overcome the difficulty of regulating the mutual interactions between the pore-forming agent and the nanocluster units, Zhang et al. introduced a micelle-directed nanocluster modular self-assembly method. By this method, the hydrogen-bonded mesoporous frameworks (HMFs) based on nanocluster building units have been synthesized for the first time by carefully manipulating the mutual interactions between the pore-forming agents of copolymer micelles and the assembly units of nanoclusters[11]. Taking the synthesis of the titanium-oxo clusters (TOCs)-based HMFs as an example, the TOCs were firstly dissolved in dichloromethane to form a well-dispersed cluster solution. Then, the amphiphilic diblock copolymer of poly(styrene-block-acrylic acid) (PS-b-PAA) was added to form a pale blue solution of PS-b-PAA/TOCs composite micelles. Afterward, these composite micelles are co-assembled to generate a uniform hexagonal transparent film with abundant hydrogen bonds during the solvent evaporation processes. Finally, the TOCs-based HMFs can be obtained by removing the micelle templates through solvent extraction [Figure 1]. Through a series of morphological and chemical characterizations of HMFs, it was demonstrated that the hydrogen bonds between the PS-b-PAA micelles and TOC are a crucial factor in controlling the formation of the HMFs.
Figure 1. (A) The formation process of the HMFs with a regular structure, including self-assembly of TOCs with block copolymer, and the high-resolution structure model of the ordered HMFs; (B) molecular model of HMFs; (C) molecular model of TOC building units; (D) chemical structure of the PS-b-PAA copolymer; and (E) the ethylene glycol ligand used to form hydrogen bond in TOCs. The red dashed line represents hydrogen bonding. Adapted from Ref.[11] with permission, copyright 2024 American Chemical Society. HMFs: Hydrogen-bonded mesoporous frameworks; TOCs: titanium-oxo clusters; PS-b-PAA: poly(styrene-block-acrylic acid).
Meanwhile, the size of the mesopores is able to be precisely changed by tuning the block lengths of PS-b-PAA copolymers. Furthermore, by altering the amount of the PS-b-PAA block copolymers contained, the mesoporous structure may be changed from spherical, short cylindrical, to long cylindrical morphologies. In addition, the micelle-directed nanocluster modular self-assembly method is universal for the synthesis of functional HMFs. Besides the TOC units, four other typical kinds of nanoclusters, including the hydrated silicotungstic acid (H4SiW12O40·xH2O), phosphomolybdic acid hydrate (H3PMo12O40·xH2O), phosphotungstic acid hydrate (H3PW12O40·xH2O), and cubane-like organometallic clusters
In order to further demonstrate that hydrogen bonds are a crucial factor for the formation of mesoporous frameworks in the micelle-directed nanocluster modular self-assembly strategy, a comparative directing copolymer micelle without surface −COOH groups, e.g., poly(styrene-block-poly(ethylene oxide)) (PS-b-PEO), was introduced to perform as a pore-forming agent. As a result, under the same synthetic condition, the obtained assemblies were poor in mesopores when directed by this PS-b-PEO micelle template. This comparative experimental result combined with the molecular dynamics (MD) simulation indicates that the porous skeleton of the nanoclusters-based HMFs is mainly stabilized through the hydrogen bonding.
For the application, taking the TOC units-based HMFs as an example, such novel HMFs were utilized as a photocatalyst for H2 production under simulated sunlight irradiation. As a comparative sample, the photocatalytic performance of the pure TOC building units was also tested. As a result, the HMF products performed better, showing a H2 evolution rate of 3.6 mmol·g-1·h-1, which is roughly twice as high as the pure TOC rate of 1.5 mmol·g-1·h-1. Simultaneously, the mesoporous framework architecture can maintain well after five times cycles, suggesting excellent photostability.
In short, Zhang et al. have demonstrated how to synthesize a novel family of mesoporous materials called HMFs using a micelle-directed nanocluster modular self-assembly approach[11]. The pore diameter can be adjusted when controlling the molecular weight of PS-b-PAA. The configuration of the mesopores can be well manipulated, ranging from the spherical, the short cylindrical to long cylindrical mesopores by controlling the concentration of PS-b-PAA. Through the general method of this modular self-assembly using nanoclusters with tunable configurations and ingredients, several new HMFs were achieved. Moreover, the hydrogen evolution was efficiently catalyzed by TOC-based HMFs (3.6 mmol·g-1·h-1), resulting in a conversion rate about twice as high as the unassembled TOCs (1.5 mmol·g-1·h-1). These findings open a new avenue for constructing nanocluster-based HMFs for various potential applications, which can attract widespread attention in the future.
DECLARATIONS
Acknowledgments
We sincerely thank all leading chemists and co-workers involved in the development of hydrogen-bonded mesoporous framework materials chemistry.
Authors’ contributions
Wrote the draft manuscript: Han X
Revised and rewrote some parts of the manuscript: Zhao Z, Zhao Y
Availability of data and materials
Further data is available from the corresponding authors upon request.
Financial support and sponsorship
We acknowledge the support from the National Key R&D Program of China (2024YFE0101100), the National Natural Science Foundation of China (22305132, 22365021, 22475112), the Inner Mongolia Autonomous Region “Grassland Talents” Project (2024098), the Inner Mongolia Natural Science Foundation Youth Fund (2023QN02014), the Basic Research Expenses Supported under 45 Years Old of Inner Mongolia (10000-23112101/036), the Local Talent Project of Inner Mongolia (12000-15042222), and the “Young academic talents” Program of Inner Mongolia University (23600-5233706).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
REFERENCES
1. Zhang S, Fu J, Xing G, Zhu W, Ben T. Recent advances in porous adsorbent assisted atmospheric water harvesting: a review of adsorbent materials. Chem Synth. 2023;3:10.
2. Liang Q, Huang Y, Guo Y, et al. Efficient osmosis-powered production of green hydrogen. Nat Sustain. 2024;7:628-39.
3. Wang J, Fan X, Han X, et al. Ultrasmall inorganic mesoporous nanoparticles: preparation, functionalization, and application. Adv Mater. 2024;36:e2312374.
4. Zhao Z, Duan L, Zhao Y, et al. Constructing unique mesoporous carbon superstructures via monomicelle interface confined assembly. J Am Chem Soc. 2022;144:11767-77.
5. Zhao Z, Liu M, Duan L, et al. Ultrafine asymmetric soft/stiff nanohybrids with tunable patchiness via a dynamic surface-mediated assembly. J Am Chem Soc. 2024;146:20857-67.
6. Yi C, Liu H, Zhang S, et al. Self-limiting directional nanoparticle bonding governed by reaction stoichiometry. Science. 2020;369:1369-74.
7. Cho SR, Kim D, Jeon M, et al. Overlaying monolayer metal–organic framework on PtSe2-based gas sensor for tuning selectivity. Adv Funct Mater. 2022;32:2207265.
8. Martín-illán JÁ, Rodríguez-San-Miguel D, Zamora F. Evolution of covalent organic frameworks: from design to real-world applications. Coord Chem Rev. 2023;495:215342.
9. Qin WK, Tung CH, Wu LZ. Covalent organic framework and hydrogen-bonded organic framework for solar-driven photocatalysis. J Mater Chem A. 2023;11:12521-38.
10. Hueckel T, Hocky GM, Palacci J, Sacanna S. Ionic solids from common colloids. Nature. 2020;580:487-90.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Author Biographies





















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].