Oxygen coverage effect promotes oxygen evolution reaction
Keywords
Green hydrogen production powered by water electrolysis stands as a promising technology for renewable energy transition and storage. However, oxygen evolution reaction (OER) with sluggish multi-electron-transferred process has limited the overall efficiency of water splitting. For iridium-based benchmark materials, understanding the intrinsic water oxidation kinetics and realizing accurate activity descriptors are key factors to help design better electrocatalysts for practical application of water electrolysis.
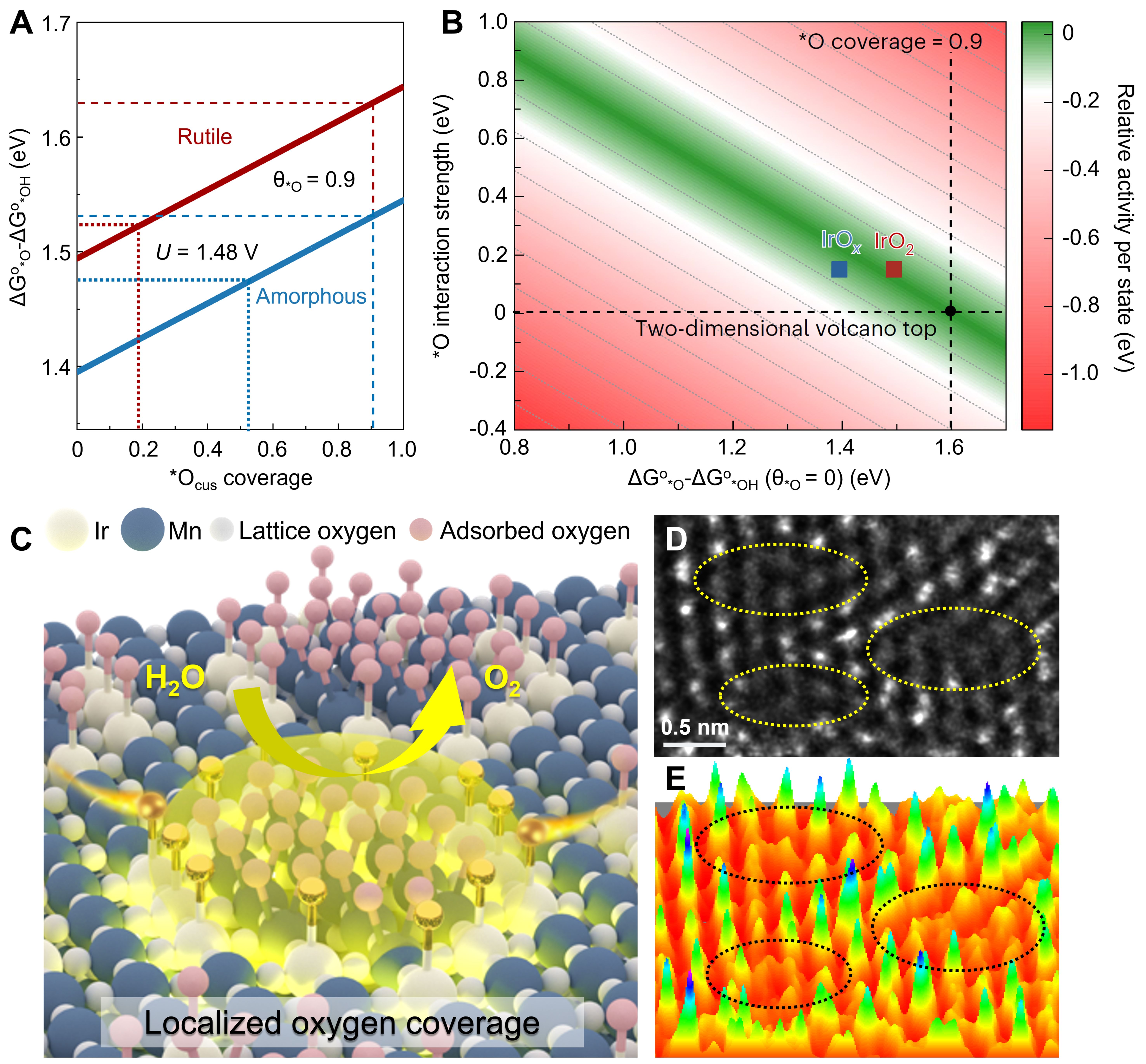
Recently reported in Nature Catalysis, through clever analysis of the absorption spectra in operando time-resolved ultraviolet-visible (UV-vis) spectroscopy, Liang et al. have quantified the active site density and oxygen binding strengths on different iridium oxides, unveiling the effect of adsorbate-adsorbate interactions on O–O bond formation[1]. Previously, for rational design of OER catalysts, oxygen adsorption energy (ΔG*O) was first introduced by Rossmeisl and Nørskov et al. to describe the OER activity[2,3], and the standard free energy change ΔG*O0 - ΔG*OH0 was universally applied as the activity descriptor with a volcano-type relationship[4-6]. In this work, besides the conventional binding energetics of ΔG*O0 - ΔG*OH0, an additional oxygen coverage effect showed how the interactions between adsorbates can control the OER kinetics [Figure 1A]. A clever modification of the conventional activity descriptor was made, as shown in the improved three-dimensional volcano plot [Figure 1B]. Accordingly, the previous descriptor ΔG*O0 -
Figure 1. (A) Experimentally determined ΔG*O0 - ΔG*OH0 values at different oxygen coverages; (B) Effects of the *O interaction strength and *O binding strength on the OER activity. Reproduced with permission[1]; (C) Schematic illustration of oxygen coverage effect on atomic grid structure toward accelerated O–O bond formation; (D and E) Atomic-resolution high-angle annular dark field scanning transmission electron microscopy image of atomic grid structure on Ir-Mn-Ov catalyst and the corresponding three-dimensional surface plot with atom-overlapping. Reproduced with permission[7].
Our recent work has also demonstrated the oxygen coverage effect to promote O–O bond formation during the OER process [Figure 1C][7]. A new metal-support configuration of dense atomic grids was constructed through high-density Ir sites (~10 atoms per nm2) supported on MnO2-x [Figure 1D and E]. Initial Mn-Ov coordination defects in MnO2-x give rise to electrochemical generation of enriched oxygen coverage as probed by the increased Mn-O coordination intensity under OER potentials from operando X-ray absorption fine structure (XAFS) spectra. Moreover, the Ir grid lines proceed with highly electrophilic nature of Ir–O(II-δ)- bonds during OER, facilitating oxygen radicals on Ir sites directly coupling with the rich oxygen adsorbates on support Mn sites. Thereby, an ultra-low OER overpotential of 166 mV at 10 mA·cm-2 and a striking mass activity which was 380 times higher than commercial IrO2 were achieved on this catalyst. The oxygen coverage effect also conforms to the practical operation at an oxygen-enriched environment on the anode side of the proton exchange membrane water electrolyzer, which leads to a low cell voltage of 1.58 V to reach the current density of 1 A·cm-2.
In summary, the oxygen coverage effect can be complementary to the conventional binding energetics. Based on this insight, future active OER catalysts can be predicted and designed far beyond the current models. Those non-precious metal-based materials, which have not been considered as promising catalysts owing to inappropriate oxygen binding strength, could be activated by optimizing oxygen coverage through crystallinity design, defect engineering, porosification treatment, and so on. Moreover, OER pathways, including enriched oxygen coverage-induced oxygen pathway mechanism (OPM), can be considered to overcome the scaling relationship between the intermediates[7]. Conventional lattice oxygen mechanism (LOM) can also be modified with high oxygen coverage, which aids in replenishing the surface oxygen vacancies that resulted from lattice oxygen oxidation and avoids structural collapse[8]. Furthermore, for impure water electrolysis such as seawater electrolysis, the oxygen coverage effect can also act as a powerful handle to prevent Cl- binding and aids the exclusive selectivity toward OER.
DECLARATIONS
Authors’ contributions
Wrote the draft manuscript: Lin H
Revised and rewrote some parts of the manuscript: Liu P, Yang H
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was financially supported by the National Natural Science Foundation of China (22239001) and the Shanghai Pilot Program for Basic Research (22TQ1400100-12).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
REFERENCES
1. Liang C, Rao RR, Svane KL, et al. Unravelling the effects of active site density and energetics on the water oxidation activity of iridium oxides. Nat Catal. 2024;7:763-75.
2. Rossmeisl J, Qu ZW, Zhu H, Kroes GJ, Nørskov JK. Electrolysis of water on oxide surfaces. J Electroanal Chem. 2007;607:83-9.
3. Rossmeisl J, Logadottir A, Nørskov JK. Electrolysis of water on (oxidized) metal surfaces. Chem Phys. 2005;319:178-84.
4. Gloag L, Somerville SV, Gooding JJ, Tilley RD. Co-catalytic metal–support interactions in single-atom electrocatalysts. Nat Rev Mater. 2024;9:173-89.
5. Deng L, Hung SF, Lin ZY, et al. Valence oscillation of Ru active sites for efficient and robust acidic water oxidation. Adv Mater. 2023;35:e2305939.
6. Oener SZ, Bergmann A, Cuenya BR. Designing active oxides for a durable oxygen evolution reaction. Nat Synth. 2023;2:817-27.
7. Lin HY, Yang QQ, Lin MY, et al. Enriched oxygen coverage localized within Ir atomic grids for enhanced oxygen evolution electrocatalysis. Adv Mater. 2024;36:e2408045.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Author Biographies



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].