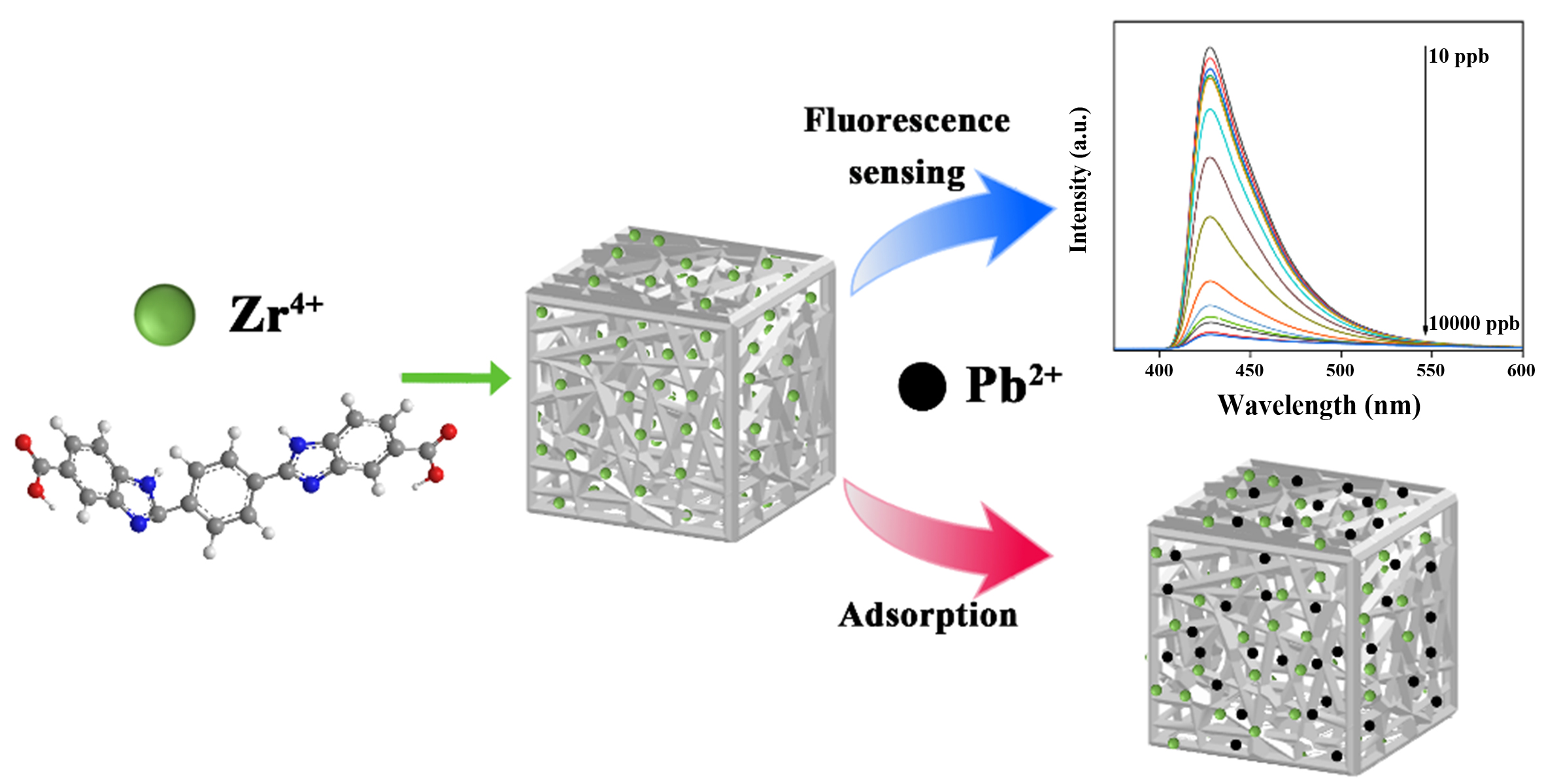
Fluorescence detection and effective adsorption of trace Pb(II) based on nanofibrous metal-organic gel
Abstract
Water pollution has become a global environmental problem, such as that caused by Pb(II). Therefore, there is an urgent need to develop multifunctional materials for Pb(II) monitoring and removal. Yet, developing bifunctional materials for sensitive detection and efficient removal of Pb(II) remain challenging. Here, a metal-organic gel (HNU-G4) was constructed for sensible responsive detection and efficient adsorption of Pb(II). The dry gel was obtained through the freeze-dried process and can be used for the Pb(II) detection via fluorescence quenching; the lowest limit of detection for Pb(II) is 0.766 ppb. Furthermore, HNU-G4 has an effective maximum adsorption capacity of 480.00 mg·g-1 for Pb(II) in water. Additionally, the gel demonstrates excellent recoverability and interference resistance, which can be used in the detection and recovery of actual reclaimed water samples to prevent secondary contamination. This study developed a bifunctional gel material for sensitive detection and effective removal of Pb(II) from water, providing a suggested strategy to tackle the heavy metal contamination problem.
Keywords
INTRODUCTION
With the rapid development of modern industry and the increase in human economic activities, heavy metal pollution has gradually become a global environmental issue[1,2]. Among the hazardous pollutants, heavy metal Pb(II) ions have received widespread attention due to their hazardous nature. The presence of Pb(II) not only threatens natural ecosystems but also poses risks to human health[3]. As it accumulates in the environment and human body, the toxic effects will affect the central nervous, digestive, reproductive, and immune systems[4]. To solve the critical issue, developing environmentally friendly materials for detecting and adsorbing heavy metal Pb(II) from water has become quite urgent.
Aimed at highly efficient detection of Pb(II), functional materials, including mesoporous nanocomposites[5], metal/covalent organic frameworks (MOFs/COFs)[6,7], and magnetic nanoparticles[8], have been developed. As a class of multifunctional materials, metal-organic gels (MOGs) have attracted widespread interest due to their excellent stabilities and unique structure. They are gel-like materials constructed through coordination between metal ions and organic molecules and non-covalent interactions including π-π stacking, hydrogen bonding, and van der Waals forces[9], which possess highly tunable structures, adjustable porosity, open active sites[10], and functional groups[11]. Benefiting from these characteristics, MOGs show a wide range of possibilities for application in the areas of catalysis[12], adsorption[13], separation[14], sensing[15], etc.
As for Pb(II) adsorption, the -NH2 group and uncoordinated N atom were adopted to improve the adsorbed amount of MOGs[16]. For example, Huang et al. used Zn to prepare an excellent amino MOF adsorbent for removing lead, mercury, and arsenic from water[17]. Separately, Yin et al. developed an effective method for removing Pb(II) by combining amino-functionalized MOFs with ceramic membrane ultrafiltration[18]. Nonetheless, the low concentration Pb(II) adsorption remains a tough problem. In addition, although the MOGs for Pb(II) adsorption have been developed, the fluorescence sensing was less explored, such as the new multifunctional gel developed by Kumar et al. and Varaprasad et al.[19,20]. Detecting the content of Pb(II) and adsorbing low concentration Pb(II) present great challenges.
By introducing functional groups and modulating the structure of MOGs, the fluorescence detection and low concentration Pb(II) adsorption could be achieved. This work adopted the organic ligand 2,2’-(1,4-phenylene)bis(1H-benzo[d]imidazole-5-carboxylic acid) (H2L1) which contains the fluorescence and coordination groups. Synthesis of metal organogels, fluorescence sensing detection and adsorption of Pb(II) were demonstrated in Scheme 1. In the ligand, the benzimidazole can serve as a fluorescence group, and the -COOH and N-H groups can serve as chelating groups for the high adsorption of Pb(II). Through the assembly of H2L1 and Zr4+, a MOG (HNU-G4) with fibrous morphology was synthesized. It should be mentioned that the minimum detection limit is 0.766 ppb, much lower than the maximum Pb(II) ion level in drinking water set by the World Health Organization (WHO)[3,4]. Furthermore, the adsorption amount of HNU-G4 for Pb(II) can reach 480.00 mg·g-1, with a removal rate of 99.98% in the trace concentration Pb(II) environment. This study provides feasible methods to address Pb(II) pollution and offers robust support and assurance for environmental protection and human health.
EXPERIMENTAL
The detailed materials and methods in the experiment were listed in the Supplementary Materials.
Synthesis of H2L1 and HNU-G4
The synthesis path for H2L1 is shown in Supplementary Figure 1A. Terephthalaldehyde (2.50 g, 0.019 mol) was added to 45 mL NaHSO3 (40% wt.) solution and sonicated for 2 h, and then 3,4-diaminobenzoic acid (5.60 g, 0.037 mol) was dispersed in 100 mL ethanol and added in the solution above slowly. The resulting mixed solution was stirred at 25 °C for 30 min followed by reflux for 5 h. In the next step, the filtered solid was washed by immersion in water and ethanol after the reaction product was cooled to 25 °C and finally recrystallized in a mixed solution [H2O:N,N-Dimethylformamide (DMF) = 1:9, v/v] to obtain H2L1. The proton magnetic resonance (1H NMR) or carbon-13 nuclear magnetic resonance (13C NMR) data of H2L1 are shown in Supplementary Figures 2 and 3.
ZrCl4 (0.05 mM), H2L1 (20 mg) and 1.5 mL DMF were placed in a 10 mL glass vial. The mixture was heated at 368 K for three days. The vials were cooled naturally to obtain a yellow wet gel, followed by freeze-drying for 24 h after washing and soaking with ethanol three times to obtain a dry gel[21]. The synthesis path for HNU-G4 is shown in Supplementary Figure 1B.
Fluorescence sensing of HNU-G4 on Pb(II)
Fluorescence measurement of Pb(II) detected by HNU-G4 was performed using the following procedure. The HNU-G4 dry gel was ground to a homogeneous powder, dispersed in water, and then sonicated for
Batch adsorption experiments
The test solution in the experiment is prepared with Pb(NO3)2. The impact of pH on adsorption was examined with Pb(II) solutions of pH = 1.0-7.0, and equilibrating pH with 0.1 mol·L-1 HCl and NaOH. HNU-G4 (3.0 mg) and Pb(II) solution (20 mg·g-1, 90 mL) were mixed well and tested at different time intervals, and the residual Pb(II) content was also determined to study the adsorption kinetics. Pb(II) solutions (90 mL) with concentrations ranging from 5.0 to 200.0 mg·g-1 were added with HNU-G4 (3.0 mg), and the residual Pb(II) content was measured to obtain adsorption isotherms. The equilibrium adsorption capacity (Qe, mg·g-1)[22] and time-dependent adsorption capacity (Qt, mg·g-1)[23,24] of HNU-G4 for Pb(II) are computed by
The Pb(II) removal rate from the solution is obtained by[25-27]
where C0 and Ct denote the initial concentration and the equilibrium concentration of Pb(II) (mg·L-1) at a certain time; Ce and Qe are the concentration and the amount of adsorption in equilibrium. V is the volume of the adsorbed environmental solution (L). m denotes the weight of HNU-G4 (g). R is the recovery rate.
Solutions containing eight metal ions [Pb(II), Ca(II), Co(II), Zn(II), Mg(II), Ni(II), K(I), and Na(I)] were used to verify the adsorption of competing coexisting ions at a solution of 20 mg·L-1. Briefly, HNU-G4
Regeneration experiments
The HNU-G4 adsorbed with Pb(II) [HNU-G4-Pb(II)] was combined with a 0.1 mol·L-1 solution of HCl. The eluate was sampled and tested for Pb(II) levels after being shaken for 2 h. Deionized water and EtOH were used for cleaning; HNU-G4 was dried at 60 °C to obtain regenerated HNU-G4. During the cyclic experiment, the process was repeated for the second cycle, which was conducted for six cycles.
RESULTS AND DISCUSSION
Characterization analysis
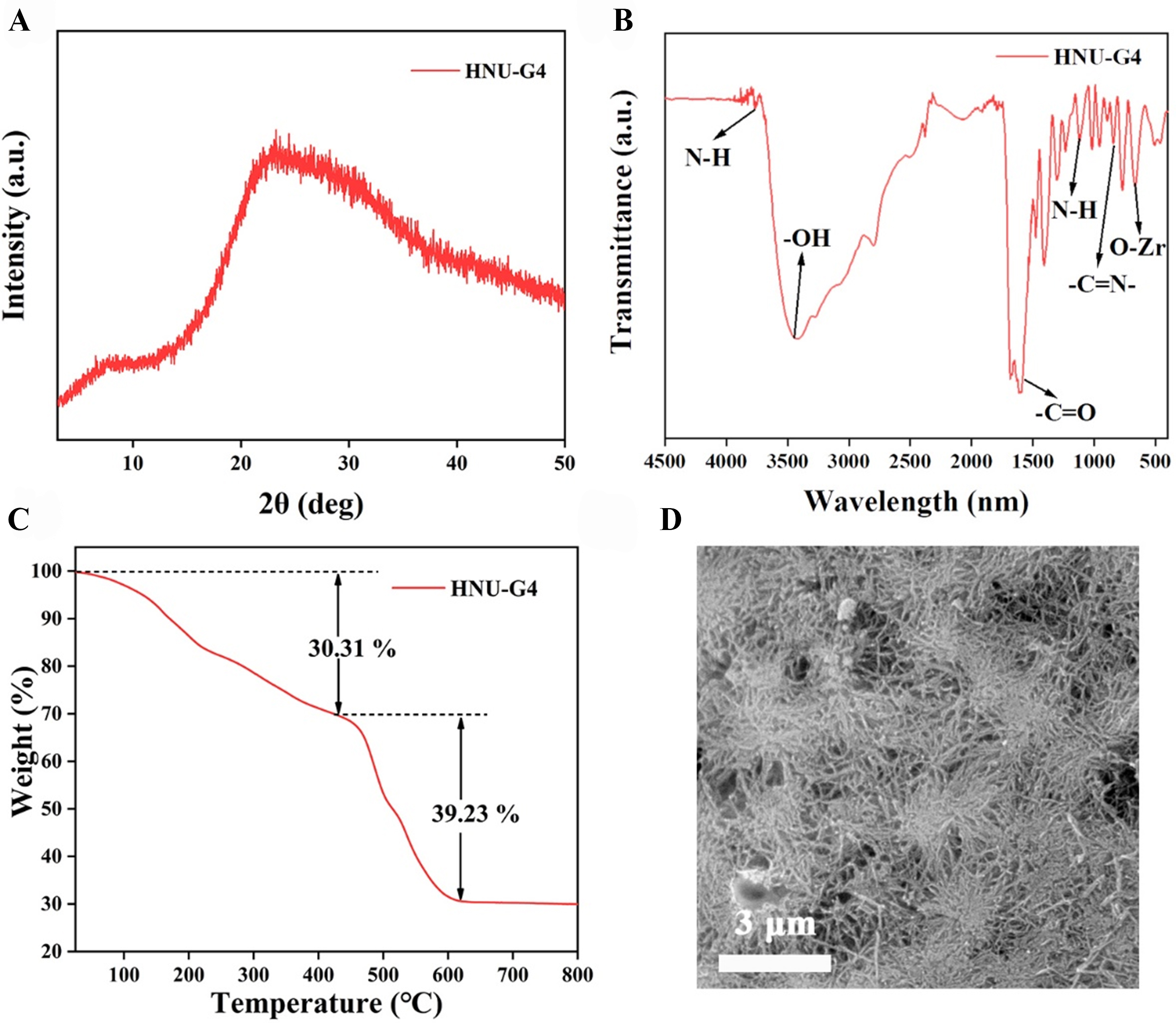
The powder X-ray diffractometer (PXRD) pattern reveals that HNU-G4 is amorphous [Figure 1A][28,29]. In Figure 1B, stretching and bending vibrations of amino N-H were observed at 3,765 and 1,109 cm-1, respectively. The peaks at 3,300-3,800 cm-1 were attributed to the stretching vibrations of -OH. Additionally, the peaks at 823 and 663 cm-1 were assigned to -C=N- and O-Zr, respectively. The thermogravimetric analysis (TGA) curve [Figure 1C] decreases significantly around 430 °C, which can be considered as the disruption of the internal structure of HNU-G4. A wet gel optical photo of HNU-G4 is shown in Supplementary Figure 4. From the scanning electron microscopy (SEM) image of the gel [Figure 1D], its surface shows a flower-like fiber structure with each fiber intertwined, which gives the gel a high specific surface area, and no clear crystalline phase is found. N2 adsorption measurements were performed at 77 K to evaluate the porosity of HNU-G4 (Supplementary Figure 5, the illustration shows the aperture distribution). The Brunauer-Emmett-Teller (BET) surface areas determined from the N2 adsorption isotherms were 4.341 m2/g.
Fluorescence sensing performance of HNU-G4 for Pb(II)
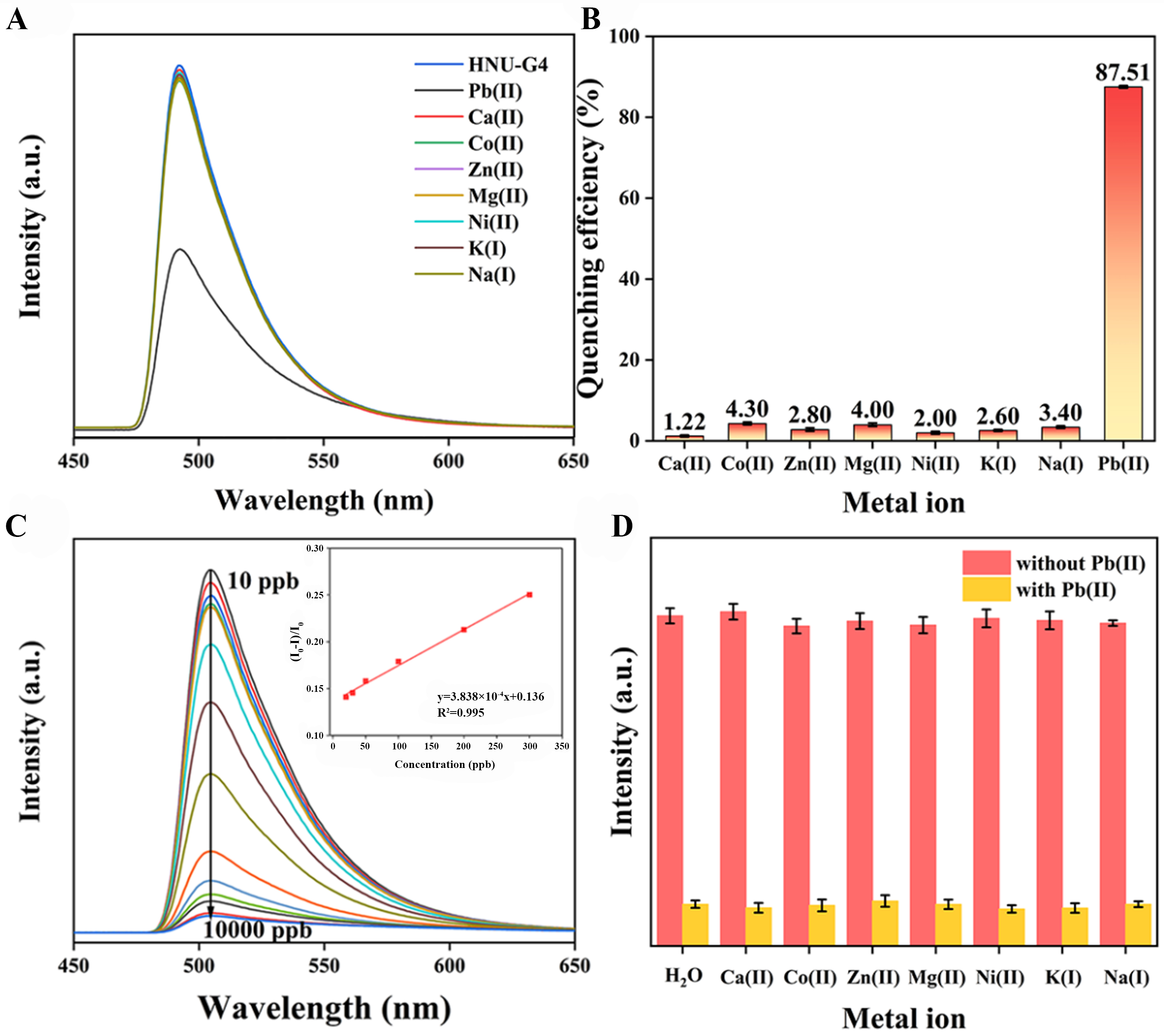
The emission spectrum of HNU-G4 in water was examined [Supplementary Figure 6], with an emission peak at 504 nm (λex = 339 nm). Additionally, the fluorescence response of HNU-G4 to common metal ions in water was tested. The Ca(II), Co(II), Zn(II), Mg(II), Ni(II), K(I), Na(I) and Pb(II) ions were introduced into the HNU-G4 suspension. The results showed that only Pb(II) showed fluorescence quenching of HNU-G4, while the existence of other common metal ions did not significantly attenuate or enhance the fluorescence about HNU-G4 [Figure 2A and B]. The quenching efficiency was calculated by[29,30]
Figure 2. Fluorescence spectra (A) and quenching efficiencies (B) of HNU-G4 by various metal ions; (C) Fluorescence intensity of HNU-G4 in different concentrations of Pb(II) solutions, the illustration shows the linear relationship at low concentrations; (D) Fluorescence intensity of HNU-G4 in mixed solutions of other single metal ions and Pb(II).
Where I0 and I are the fluorescence intensity of HNU-G4 before and after mixing Pb(II). The fluorescence intensity of HNU-G4 was attenuated by up to 87.51% after the addition of Pb(II) [Figure 2B], while the quenching efficiency of other metal ions for HNU-G4 was less than 5%, and the results showed that the selectivity for detecting other metal ions was much lower than Pb(II). The sensitivity of HNU-G4 to Pb(II) was investigated by concentration-dependent experiments. Photoluminescence (PL) emission spectra
In addition, the limit of detection (LOD) (3σ/K)[31-33] was calculated from the K value (K = 0.0383) and standard deviation (σ) of the fluorescence intensity of the blank samples. The calculated LOD was 0.766 ppb, comparable to or below the reported results [Table 1]. The low LOD can be attributed to chelation quenched fluorescence (CHQF) of HNU-G4. To verify the selectivity of HNU-G4 for Pb(II), further competition experiments were performed [Figure 2D], where the presence of other common metal ions did not interfere with the reactivity of HNU-G4 to Pb(II). These results reveal that HNU-G4 exhibits specificity in detecting Pb(II).
Comparison of adsorption capacity, detection methods and detection limits for HNU-G4 and other adsorbents
| Adsorbents | Adsorption capacity (mg·g-1) | Detection method | LOD (ppb) | Ref. |
| BUC-77 | 425.00 | Fluorescence | 6.910 | [34] |
| IIMB | 118.00 | FAAS | 0.950 | [35] |
| Triplochyton scleroxylon | 298.00 | Electrochemical | 0.820 | [36] |
| mesoporous adsorbent | 184.32 | UV-vis-NIR | 0.110 | [15] |
| PCM | 204.34 | UV-vis spectrophotometer | 0.330 | [37] |
| UiO-66-NHC(S)NHMe | 391.00 | Electrochemical | 2.300 | [38] |
| P(TA-MBA-GGQDs)/Ge DN gel | 259.067 | Naked-eye and fluorescence | 0.052 | [16] |
| Functional composite material | 176.66 | UV-vis | 0.440 | [39] |
| Flower-like NiO/rGO nanocomposite | 70.00 | SWASV | 2.070 | [40] |
| HNU-G4 | 480.00 | Fluorescence | 0.766 | This work |
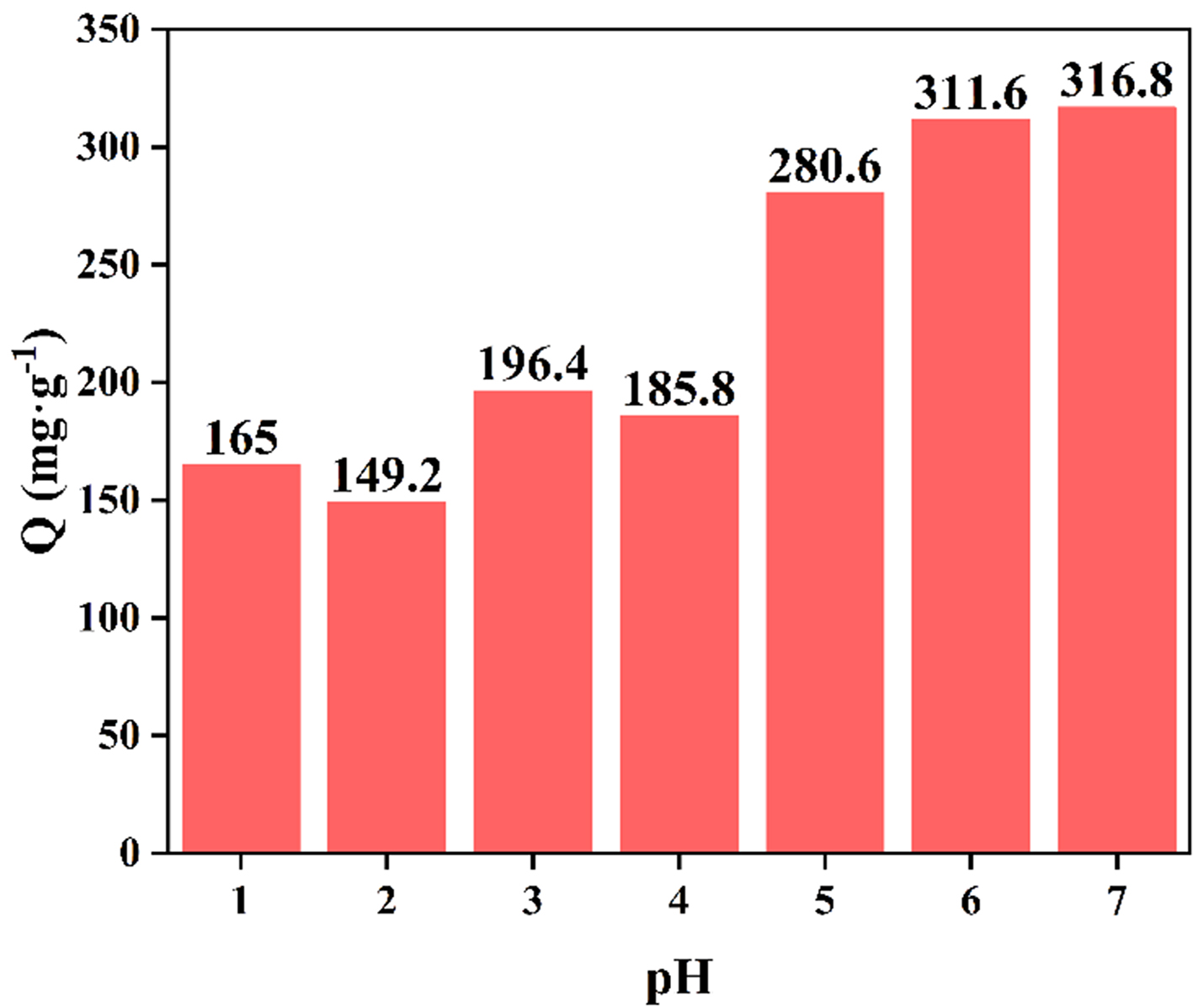
Effect of pH in structure and adsorption
Structural stability is a critical consideration for evaluating the application of MOGs, so that of HNU-G4 was investigated in acidic solutions. HNU-G4 was tested after immersing it in solutions with pH = 1.0-7.0 for 12 h. No significant peaks of disappearance or displacement were observed in its PXRD patterns
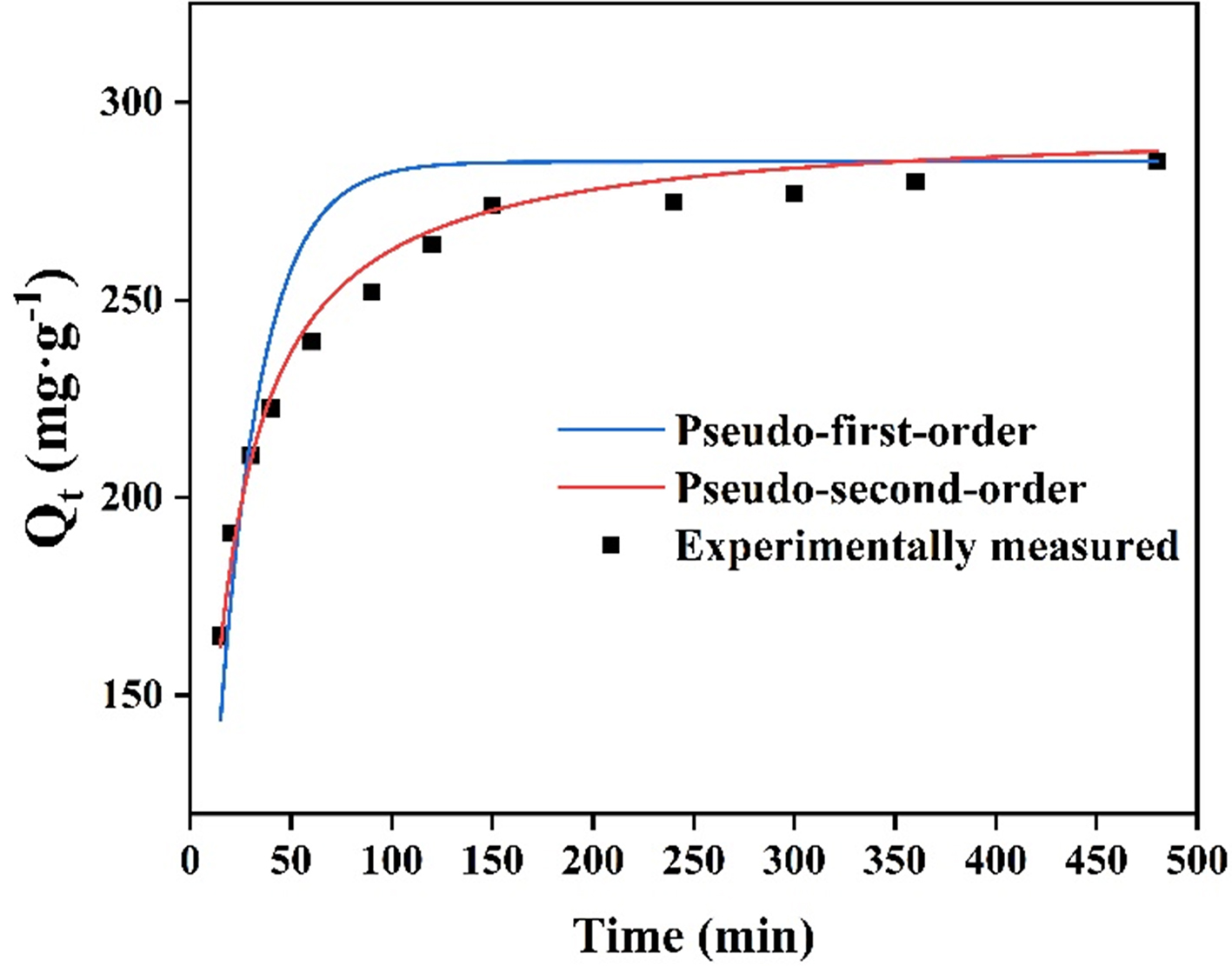
Adsorption kinetics study
From 0 to 90 min, the adsorption curve reveals a sharp increase in adsorption. The adsorption curve slowly rises with time and reaches equilibrium after 120 min [Figure 4]. Modeling the adsorption kinetics allowed a more thorough investigation of the adsorption mechanism[42]. The parameters were provided in Supplementary Table 1, and the pseudo-first-order and pseudo-second-order kinetics were fitted independently [Figure 4]. With an adsorption of 298.26 mg·g-1, consistent with the experimental test data (296.88 mg·g-1), the experimental data are more in line with the pseudo-second-order model. The primary factor in the fit to the pseudo-second-order adsorption kinetics was the chemisorption adsorption curve during the adsorption process.
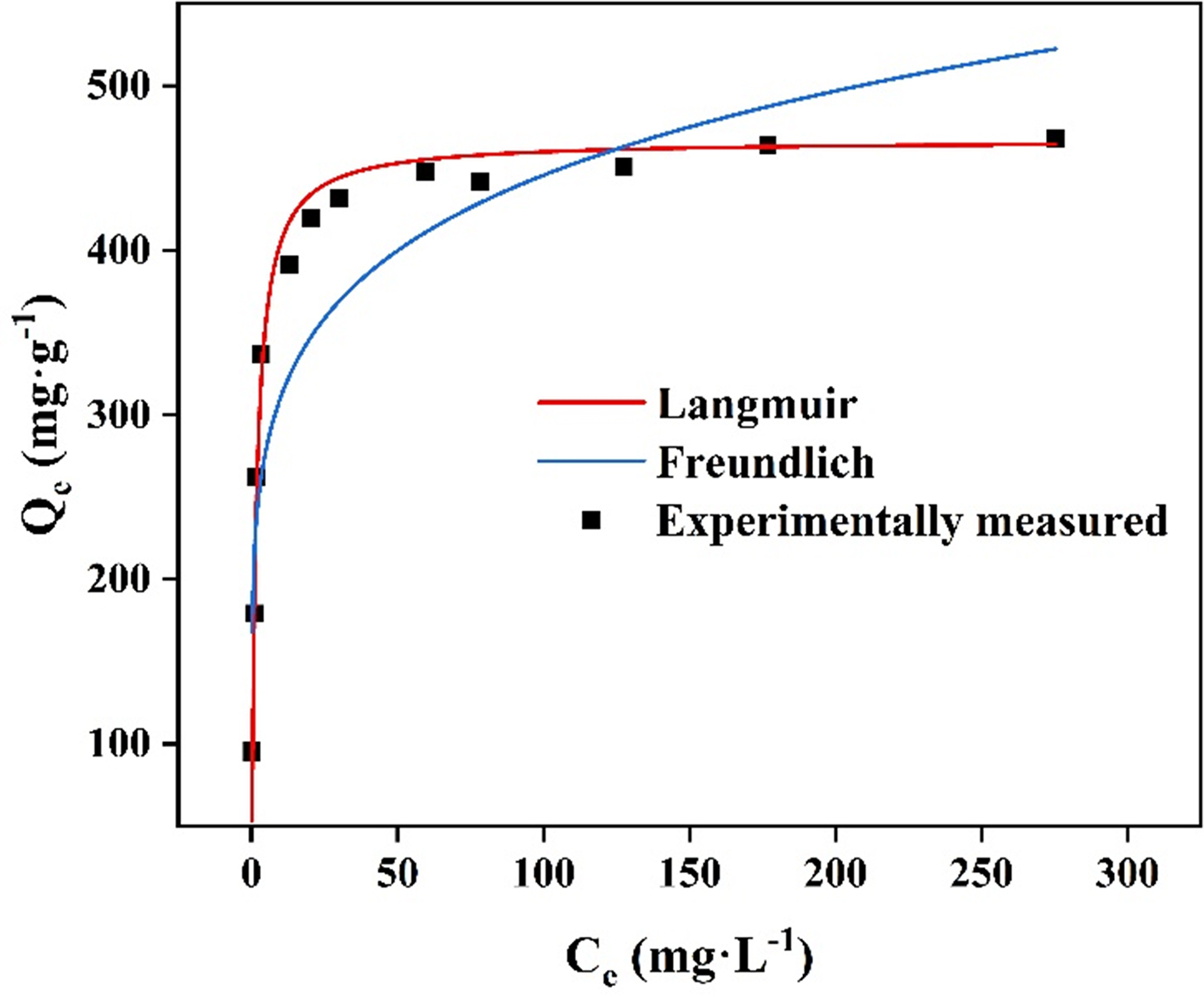
Adsorption isotherm study
The Langmuir and Freundlich isotherm models were applied to match the adsorption data, and the fitting results of the experimental data are shown in Figure 5 with detailed parameters in Supplementary Table 2. The experimental data matched the Langmuir model more closely. The estimated value of 468.00 mg·g-1 can be compared to the actual adsorption amount (468.05 mg·g-1). Since HNU-G4 possesses a homogeneous distribution of adsorption sites, the adsorption method it uses to bind Pb(II) is monolayer adsorption. Compared to other adsorbents, the HNU-G4 developed in this study showed superior performance in adsorbing heavy metal ions. The adsorption performance of the different types of adsorbents is summarized in Table 1. In comparison, the HNU-G4 in this research performs better in adsorbing Pb (II).
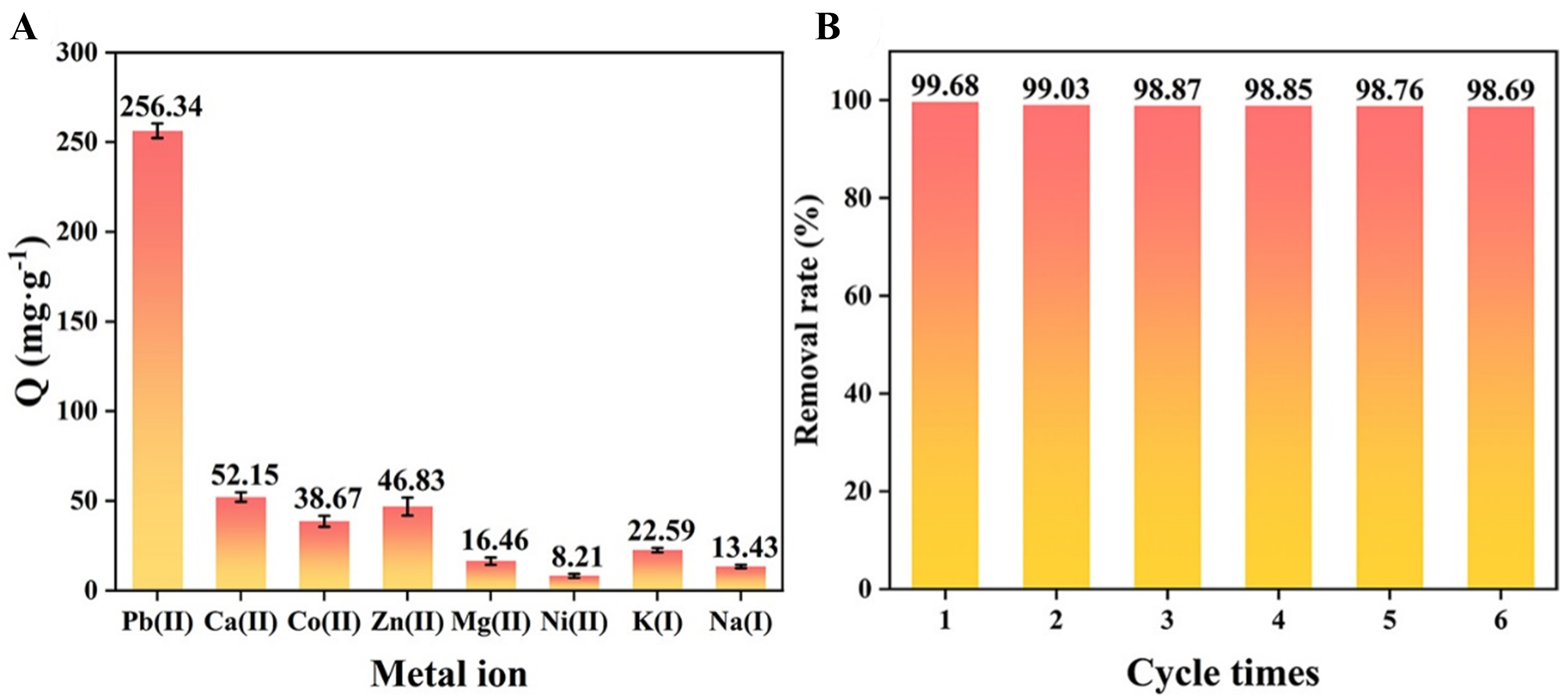
Selectivity and reusability
Commonly used metal ions [Fe(III), Al(III), Zn(II), Cu(II)), Cd(II), Mn(II), Ca(II), Ni(II), Na(I), K(I)] were used to study the selectivity of Pb(II) adsorption by HNU-G4. In Figure 6A, the adsorption capacity of HNU-G4 on Pb(II) was stronger than that of other metal ions. To investigate the ability of HNU-G4 to adsorb Pb(II) in a multicomponent system, commonly used metal ions were selected as competing ions, and Supplementary Figure 9 displays the HNU-G4 ions in the competitive ion multicomponent system. The findings showed that Pb(II) was removed from the multicomponent system by HNU-G4 at a rate higher than that of other common metals and that other ions had no effect on Pb(II) adsorption by HNU-G4. As displayed in Figure 6B, the removal rate of Pb(II) by HNU-G4 did not decrease significantly after six cycles of experiments, and the removal efficiency remained above 98.68%.
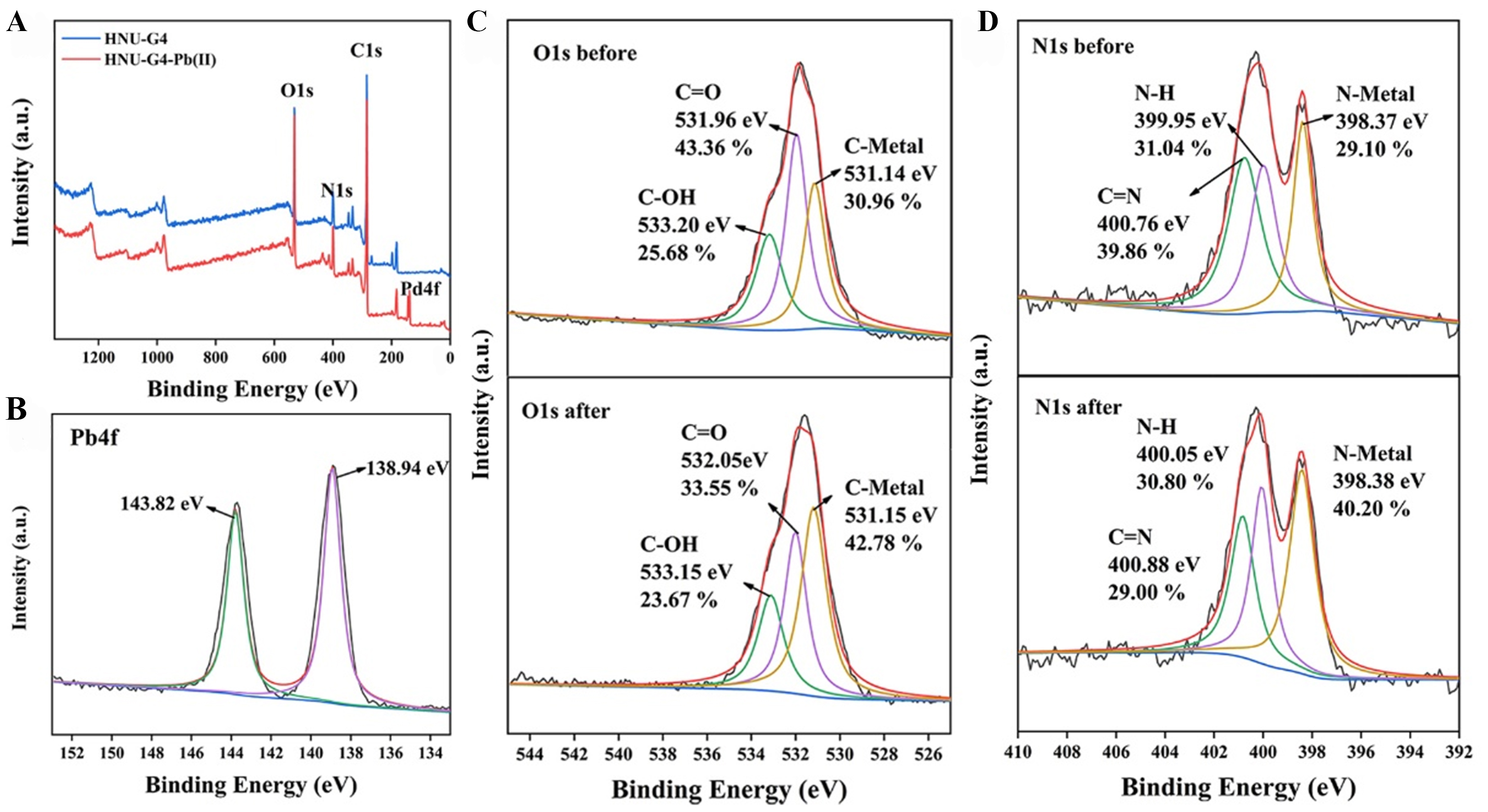
Possible sensing and adsorption mechanisms
The element mapping of blank gel and the element atlas after immersion in Pb (II) solution are shown in Supplementary Figure 10, proving that Pb is adsorbed on HNU-G4. Based on these results, a possible mechanism of the fluorescence detection and adsorption by HNU-G4 on Pb(II) was proposed. Specifically, electron-rich carboxyl and N-H in organic ligands tend to donate electrons to the 6p-orbital of Pb(II) with vacant electrons[16]. Hence, the fluorescence sensing of Pb(II) by HNU-G4 was attributed to CHQF[16]. On the other hand, Pb(II) can be chelated with the reactive groups of the gel to effectively adsorb Pb(II)[43,44]. The peaks of Pb4f 7/2 and Pb4f 5/2 at 138.94 and 143.82 eV for HNU-G4-Pb(II) indicate that Pb(II) is adsorbed on the material, as shown in the X-ray photoelectron spectroscopy (XPS) spectra
Adsorption of Pb(II) to reclaimed water
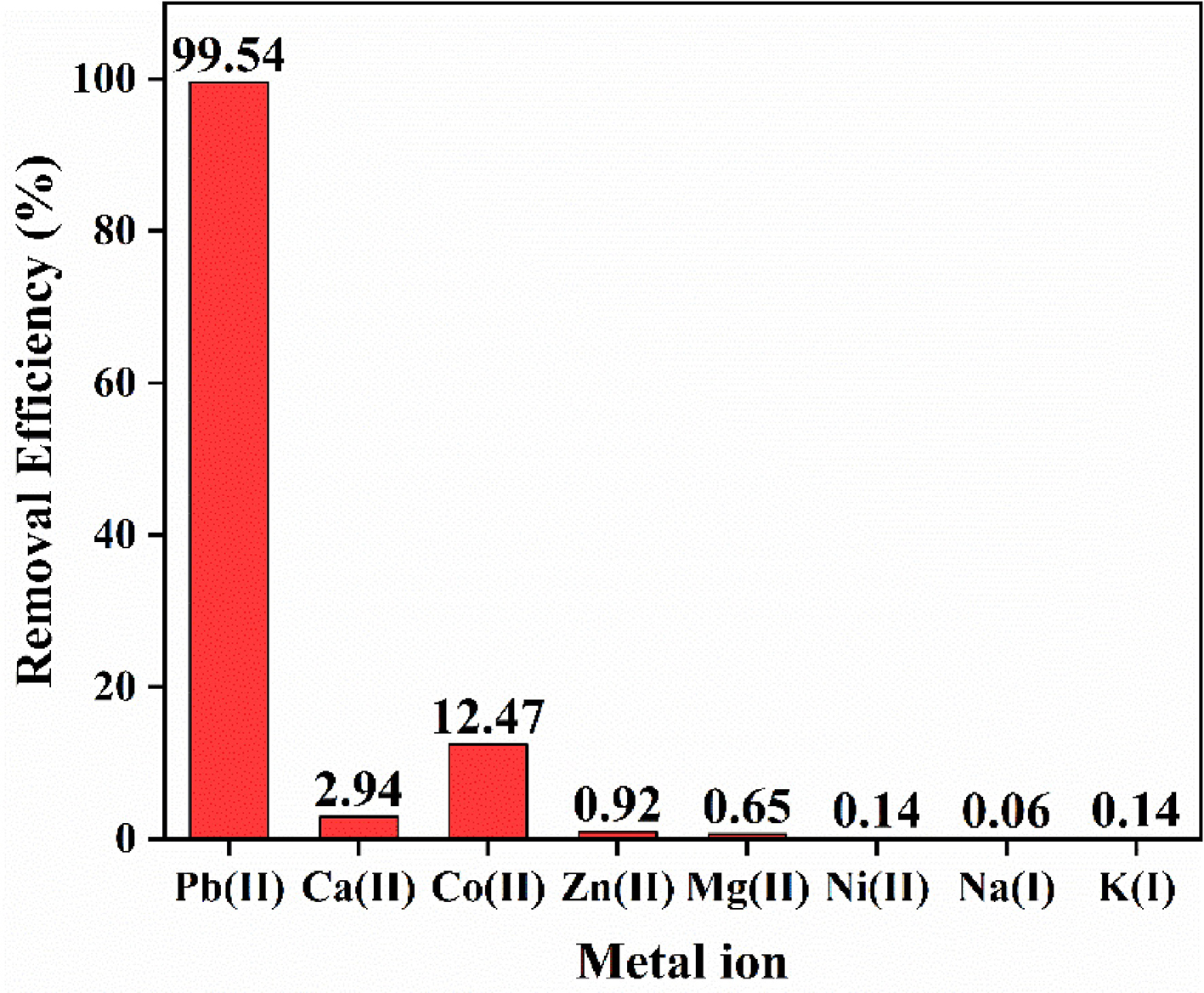
The application with HNU-G4 in practice was verified through adsorption experiments using it on reclaimed water from regenerated water treatment plants. Before and after the adsorption experiment with HUN-G4, the concentrations of various metal ions are shown in Supplementary Table 3, and the experimental data were tested by ICP; the removal rate was calculated using Equation (3). HNU-G4 had an up to 99.54% removal efficiency for Pb(II), but less than 15% of other common metal ions were removed, and the removal of Pb(II) by HNU-G4 was unaffected by the presence of other common metal ions [Figure 8]. The experiment demonstrated that Pb(II) removal with HNU-G4 in medium water was significantly faster than removal of other common metal ions. HNU-G4 was, therefore, employed as an adsorbent to remove Pb(II) from the real samples.
CONCLUSIONS
In summary, a bifunctional gel (HNU-G4) with high sensitivity and selectivity to detect and remove trace amounts of Pb(II) was developed in this work with a minimum detection limit (0.766 ppb) well below the WHO permission. Additionally, the larger the specific surface area, the more adsorption sites on the surface. The fiber structure of HNU-G4 provides a larger specific surface area. Therefore, its adsorption of Pb(II) was allowed to reach 480.00 mg·g-1, and the adsorption amount is higher than or comparable to the reported results. After six rounds of detection, adsorption, and regeneration, the gel demonstrated good reusability. Furthermore, it demonstrated good applicability in the Pb(II) detection in real media reclaimed water samples, which has a lot of promise for use in real-world scenarios. This work provides a new design strategy for the detection and adsorption of Pb(II) and suggestions for developing new multifunctional functional materials for monitoring and removing Pb(II) contamination.
DECLARATIONS
Authors’ contributions
Designed the experiments, performed data acquisition, writing - original draft preparation, formal analysis: Fang Y
Supervision, investigation, visualization, writing - review and editing: Ren G, Pan Q
Validation, formal analysis: Yu K, Wang C, Zhou Q, Che G, Li M
Availability of data and materials
The detailed materials and methods in the experiment were listed in the Supplementary Materials. Other raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This study received funding from several sources, including the National Natural Science Foundation of China (Nos. 22361016 and 22361017), the Natural Science Foundation of Hainan Province (No. 221RC451), the Education Department of Hainan Province (No. Hnky2022ZD-3), the Innovation Platform for Academicians of Hainan Province, and the Specific Research Fund of the Innovation Platform for Academicians of Hainan Province (No. YSPTZX202321), and the International Science & Technology Cooperation Program of Hainan Province (No. GHYF2022006).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
1. Wang Z, Li TT, Peng HK, Ren HT, Lou CW, Lin JH. Low-cost hydrogel adsorbent enhanced by trihydroxy melamine and β-cyclodextrin for the removal of Pb(II) and Ni(II) in water. J Hazard Mater. 2021;411:125029.
2. Jasim SA, Hachem K, Abdelbasset WK, Yasin G, Suksatan W, Chem C. Efficient removal of Pb(II) using modified chitosan Schiff base@Fe/NiFe. Int J Biol Macromol. 2022;204:644-51.
3. Rajput S, Pittman CU Jr, Mohan D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J Colloid Interface Sci. 2016;468:334-46.
4. Ozdes D, Gundogdu A, Kemer B, Duran C, Senturk HB, Soylak M. Removal of Pb(II) ions from aqueous solution by a waste mud from copper mine industry: equilibrium, kinetic and thermodynamic study. J Hazard Mater. 2009;166:1480-7.
5. Zhang J, Li L, Li Y, Yang C. Microwave-assisted synthesis of hierarchical mesoporous nano-TiO2/cellulose composites for rapid adsorption of Pb2+. Chem Eng J. 2017;313:1132-41.
6. Li G, Ye J, Fang Q, Liu F. Amide-based covalent organic frameworks materials for efficient and recyclable removal of heavy metal lead (II). Chem Eng J. 2019;370:822-30.
7. Huang Z, Xiong C, Ying L, et al. A post-functional Ti-based MOFs composite for selective removal of Pb (II) from water. J Hazard Mater. 2022;432:128700.
8. Wang P, Shen T, Li X, Tang Y, Li Y. Magnetic mesoporous calcium carbonate-based nanocomposites for the removal of toxic Pb(II) and Cd(II) ions from water. ACS Appl Nano Mater. 2020;3:1272-81.
9. Lu S, Huang J, Liu G, et al. Ammonia-modulated reversible gel-solution phase transition and fluorescence switch for a salicylhydrazide-based metal-organic gel. RSC Adv. 2017;7:30979-83.
10. Liu W, Yang Y, Zhong Q, et al. Zr4+-based metal organic gel as a fluorescent “Turn on-off” sensing platform for the selective detection and adsorption of CrO42-. Mater Chem Front. 2021;5:1932-41.
11. You D, Shi H, Xi Y, et al. Simultaneous heavy metals removal via in situ construction of multivariate metal-organic gels in actual wastewater and the reutilization for Sb(V) capture. Chem Eng J. 2020;400:125359.
12. Wang H, Chen BH, Liu DJ. Metal-organic frameworks and metal-organic gels for oxygen electrocatalysis: structural and compositional considerations. Adv Mater. 2021;33:e2008023.
13. Gao Y, Liu Z, Li Y, Zou D. Three-in-one multifunctional luminescent metal-organic gels/sodium alginate beads for high-performance adsorption and detection of chlortetracycline hydrochloride, and high-security anti-counterfeiting. Chem Eng J. 2023;452:139194.
14. Ma Y, Li A, Gao X, et al. Effective separation of enantiomers based on novel chiral hierarchical porous metal-organic gels. Macromol Rapid Commun. 2019;40:e1800862.
15. Qi H, Zhang T, Jing C, et al. Metal-organic gel as a fluorescence sensing platform to trace copper(II). Anal Methods. 2021;14:52-7.
16. Zhang K, Dai Z, Zhang W, et al. EDTA-based adsorbents for the removal of metal ions in wastewater. Coord Chem Rev. 2021;434:213809.
17. Huang Z, Zhao M, Wang C, Wang S, Dai L, Zhang L. Preparation of a novel Zn(II)-imidazole framework as an efficient and regenerative adsorbent for Pb, Hg, and As ion removal from water. ACS Appl Mater Interfaces. 2020;12:41294-302.
18. Yin N, Wang K, Wang L, Li Z. Amino-functionalized MOFs combining ceramic membrane ultrafiltration for Pb (II) removal. Chem Eng J. 2016;306:619-28.
19. Kumar A, Chowdhuri AR, Laha D, Mahto TK, Karmakar P, Sahu SK. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sensor Actuat B Chem. 2017;242:679-86.
20. Varaprasad K, Nùñez D, Ide W, Jayaramudu T, Sadiku ER. Development of high alginate comprised hydrogels for removal of Pb(II) ions. J Mol Liq. 2020;298:112087.
21. Chen JF, Liu X, Ma JF, et al. A pillar[5]arene-based multiple-stimuli responsive metal-organic gel was constructed for facile removal of mercury ions. Soft Matter. 2017;13:5214-8.
22. Chen J, Dong R, Chen S, et al. Selective adsorption towards heavy metal ions on the green synthesized polythiophene/MnO2 with a synergetic effect. J Clean Prod. 2022;338:130536.
23. Wang Y, van Zwieten L, Wang H, et al. Sorption of Pb(II) onto biochar is enhanced through co-sorption of dissolved organic matter. Sci Total Environ. 2022;825:153686.
24. Fu L, Wang S, Lin G, et al. Post-modification of UiO-66-NH2 by resorcyl aldehyde for selective removal of Pb(II) in aqueous media. J Clean Prod. 2019;229:470-9.
25. Ahmadijokani F, Tajahmadi S, Bahi A, et al. Ethylenediamine-functionalized Zr-based MOF for efficient removal of heavy metal ions from water. Chemosphere. 2021;264:128466.
26. Saleem H, Rafique U, Davies RP. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Micropor Mesopor Mater. 2016;221:238-44.
27. Tang J, Chen Y, Zhao M, Wang S, Zhang L. Phenylthiosemicarbazide-functionalized UiO-66-NH2 as highly efficient adsorbent for the selective removal of lead from aqueous solutions. J Hazard Mater. 2021;413:125278.
28. Zhang YW, Cao Y, Mao CJ, Jiang D, Zhu W. An iron(III)-based metal-organic gel-catalyzed dual electrochemiluminescence system for cytosensing and in situ evaluation of the VEGF165 subtype. Anal Chem. 2022;94:4095-102.
29. Gu D, Yang W, Lin D, et al. Water-stable lanthanide-based metal-organic gel for the detection of organic amines and white-light emission. J Mater Chem C. 2020;8:13648-54.
30. Fang Y, Ren G, Li M, Yang Y, Guo D, Pan Q. Sensitively liquid and gaseous detection of formaldehyde based on a supramolecular organic framework. Sensor Actuat B Chem. 2021;349:130726.
31. Sun Y, Dramou P, Song Z, et al. Lanthanide metal doped organic gel as ratiometric fluorescence probe for selective monitoring of ciprofloxacin. Microchem J. 2022;179:107476.
32. Zhang Y, Li B, Ma H, Zhang L, Zhang W. An RGH-MOF as a naked eye colorimetric fluorescent sensor for picric acid recognition. J Mater Chem C. 2017;5:4661-9.
33. Afzalinia A, Mirzaee M. Ultrasensitive fluorescent miRNA biosensor based on a “sandwich” oligonucleotide hybridization and fluorescence resonance energy transfer process using an Ln(III)-MOF and Ag nanoparticles for early cancer diagnosis: application of central composite design. ACS Appl Mater Interfaces. 2020;12:16076-87.
34. Song XX, Fu H, Wang P, Li HY, Zhang YQ, Wang CC. The selectively fluorescent sensing detection and adsorptive removal of Pb2+ with a stable [δ-Mo8O26]-based hybrid. J Colloid Interface Sci. 2018;532:598-604.
35. Wu P, He Y, Lu S, et al. A regenerable ion-imprinted magnetic biocomposite for selective adsorption and detection of Pb2+ in aqueous solution. J Hazard Mater. 2021;408:124410.
36. Ngana BN, Seumo PMT, Sambang LM, Dedzo GK, Nanseu-njiki CP, Ngameni E. Grafting of reactive dyes onto lignocellulosic material: application for Pb(II) adsorption and electrochemical detection in aqueous solution. J Environ Chem Eng. 2021;9:104984.
37. Awual MR. Innovative composite material for efficient and highly selective Pb(II) ion capturing from wastewater. J Mol Liq. 2019;284:502-10.
38. Pei L, Yang H, Chen S, Wang L. UiO-66-NHC(S)NHMe/three-dimensional macroporous carbon for removal and electrochemical detection of Cd2+, Pb2+, Cu2+, and Hg2+. Ind Eng Chem Res. 2022;61:1588-95.
39. Awual MR, Hasan MM, Iqbal J, et al. Naked-eye lead(II) capturing from contaminated water using innovative large-pore facial composite materials. Microchem J. 2020;154:104585.
40. Sun Y, Jian-wang, Li P, Yang M, Huang X. Highly sensitive electrochemical detection of Pb(II) based on excellent adsorption and surface Ni(II)/Ni(III) cycle of porous flower-like NiO/rGO nanocomposite. Sensor Actuat B Chem. 2019;292:136-47.
41. Schecher WD, Mcavoy DC. MINEQL+: a software environment for chemical equilibrium modeling. Comput EnvironUrban Syst. 1992;16:65-76.
42. Qi N, Zhao H, Qin Y, Wang Q, Wang G, Li Y. An innovative strategy for synchronous treatment of combined heavy metal and organic pollutants through polysaccharide gel encapsulating S2. Sci Total Environ. 2020;742:140601.
43. Ha HD, Jang M, Liu F, Cho Y, Seo TS. Upconversion photoluminescent metal ion sensors via two photon absorption in graphene oxide quantum dots. Carbon. 2015;81:367-75.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].