fig8
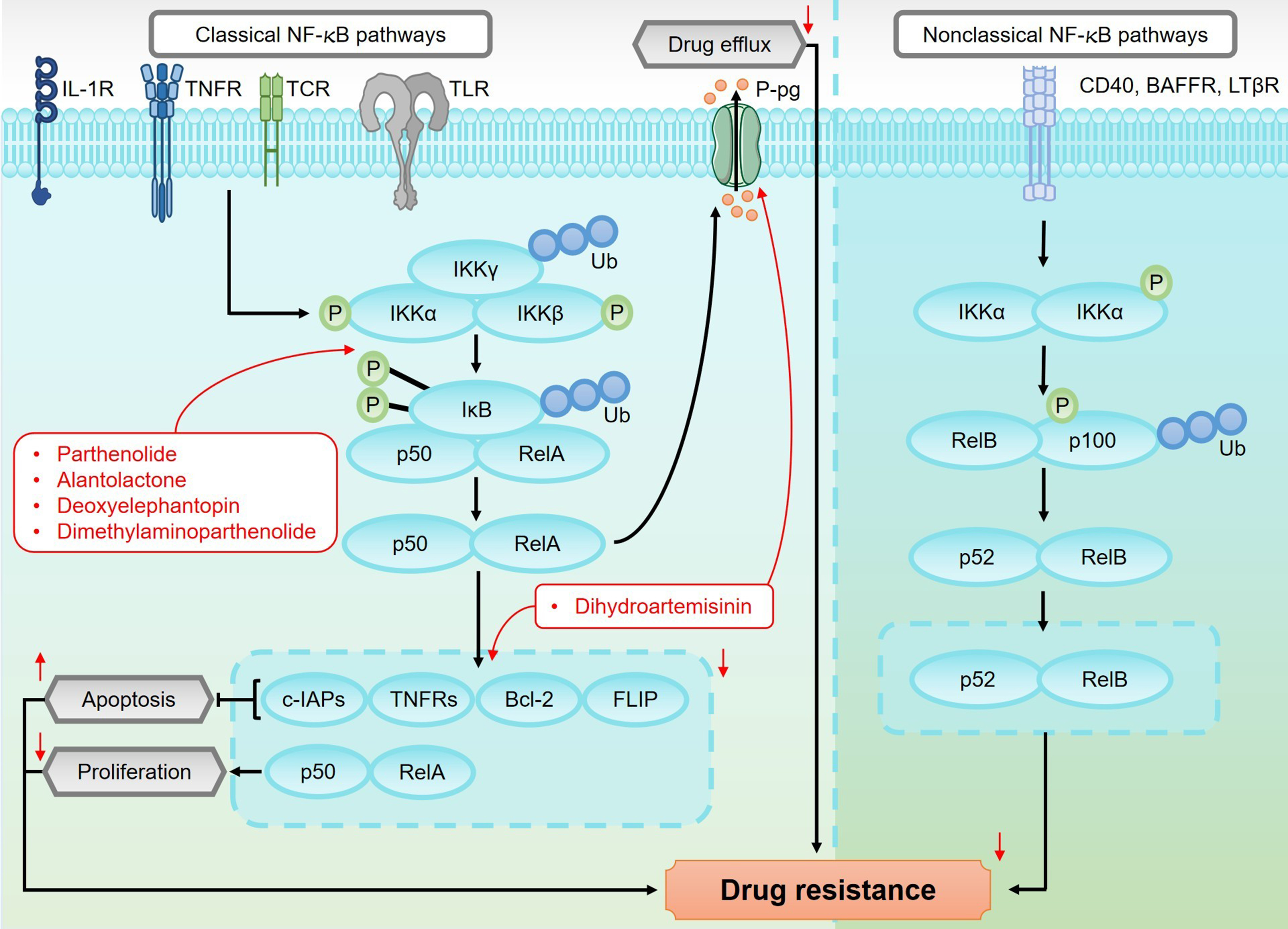
Figure 8. An overview of the canonical and non-canonical NF-κB pathways and their roles in regulating drug resistance in sesquiterpene lactones. The canonical NF-κB pathway is activated by pro-inflammatory cytokines and stress signals, which trigger the phosphorylation of IKKs. This phosphorylation leads to the degradation of IκB proteins, releasing NF-κB dimers, such as p50/p65. These dimers then translocate to the nucleus, where they regulate the expression of genes involved in inflammation, immune response, and tumor progression. In contrast, the non-canonical NF-κB pathway is activated by specific members of the TNF receptor superfamily, such as BAFFR, CD40, and LTβR. This activation leads to the activation of NIK, which in turn activates IKKα. IKKα phosphorylates p100, resulting in its partial degradation to generate p52. The p52/RelB complex then translocates to the nucleus, where it regulates genes associated with immunity and tumorigenesis. Activation of the canonical NF-κB pathway promotes the upregulation of P-gp, which enhances drug efflux and contributes to drug resistance. Additionally, NF-κB activation increases the expression of anti-apoptotic proteins such as FLIP, c-IAPs, TNFRs, and Bcl-2. These proteins inhibit apoptosis, thereby promoting chemotherapy resistance. NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; IKKs: IκB kinases; TNF: tumor necrosis factor; NIK: NF-κB-inducing kinase; P-gp: P-glycoprotein; FLIP: FLICE-inhibitory protein; TNFRs: tumor necrosis factor receptors.












