Robotic caudo-peripheral approach for liver parenchymal transection in anatomical liver resections for hepatocellular carcinoma
Abstract
Liver parenchymal transection is a challenging step during hepatic resection, particularly when using robotic platforms that require specific skills to optimize this phase. Pedicle division at the beginning of the liver parenchyma helps to better identify the resection plane and minimizes blood loss. The three-dimensional (3D) high-definition vision and the robotic Maryland allow for clear identification of the hepatic pedicles that could be dissected or divided without the need for a laparoscopic ultrasonic dissector. The caudo-peripheral technique, combined with the Maryland bipolar Kelly clamp crushing technique, is a useful approach to complete parenchymal transection and achieve safe anatomical resections in cases of hepatocellular carcinoma (HCC) with multi-pronged bleeding control. This is essential for expediting the procedure, reducing the number of intermittent clamping times, and minimizing the risk of ischemia-reperfusion injury. In this setting, perfect synchronization between the surgeon operating at the console and the bedside assistant is crucial. Advances in artificial intelligence (AI) systems have shown great potential to redefine clinical care management, preoperative planning, and intraoperative decision making for patients with HCC. This paper describes the most relevant details of our technique, its theoretical background, advantages, and limitations. Moreover, minimally invasive surgery offers the opportunity to share surgical experiences and technical progress through multimedia videos. This represents a modern and effective teaching tool to accelerate the learning process and overcome the challenges of the most complex procedures by offering surgeons various solutions to common technical problems.
Keywords
INTRODUCTION
Liver parenchymal transection is one of the key steps in performing an anatomical resection. It should ensure an optimal resection margin while minimizing blood loss. Several techniques have been described for open, laparoscopic and, more recently, robotic resections[1]. At the state of the art, robotic transection of the liver parenchyma can be achieved with two different approaches: (a) Robotic Assisted, with transection performed by the bedside surgeon using laparoscopic instruments not belonging to the robotic system but under robotic assistance (waterjet, cavitron ultrasonic surgical aspirator); (b) Totally Robotic, where liver transection is carried out with dedicated robotic tools without peculiar laparoscopic instruments[2].
The presence of hepatocellular carcinoma (HCC) adds several challenges to the surgeon on multiple fronts: technical, organizational, and training. The first challenge is the patients’ selection, which represents the most critical prerequisite to achieving optimal outcomes. The second arises from two seemingly opposing technical and oncological requirements that apply to HCC surgery: the need for anatomical resections and parenchyma preservation. Given the HCC’s tendency to spread through the portal system, resection of the tumoral portal area according to segmental anatomy is demanded in order to avoid local recurrence and improve survival. However, patients with underlying chronic liver disease have a limited hepatic functional reserve and preserving healthy parenchyma is mandatory to prevent liver failure, the main cause of postoperative mortality. Therefore, many surgeons resort to non-anatomical resections, prioritizing parenchymal sparing. A further non-negligible challenge posed by minimally invasive approaches is the steep learning curve and the need for well-trained personnel across various roles, including anesthetists, nurses, and operating room staff, who are crucial for supporting surgeons during the early learning phase. This comprehensive support contributes to improved operative time, intraoperative complication management, blood loss, and conversion rates. As a result, this surgery remains less reproducible and confined to a few expert centers.
In our Department, we developed a caudo-peripheral method for parenchymal liver transection by combining different techniques learned from laparoscopic resections and applied them to the robotic approach.
HOW WE DO IT: TIPS AND TRICKS
Patient selection and preoperative planning
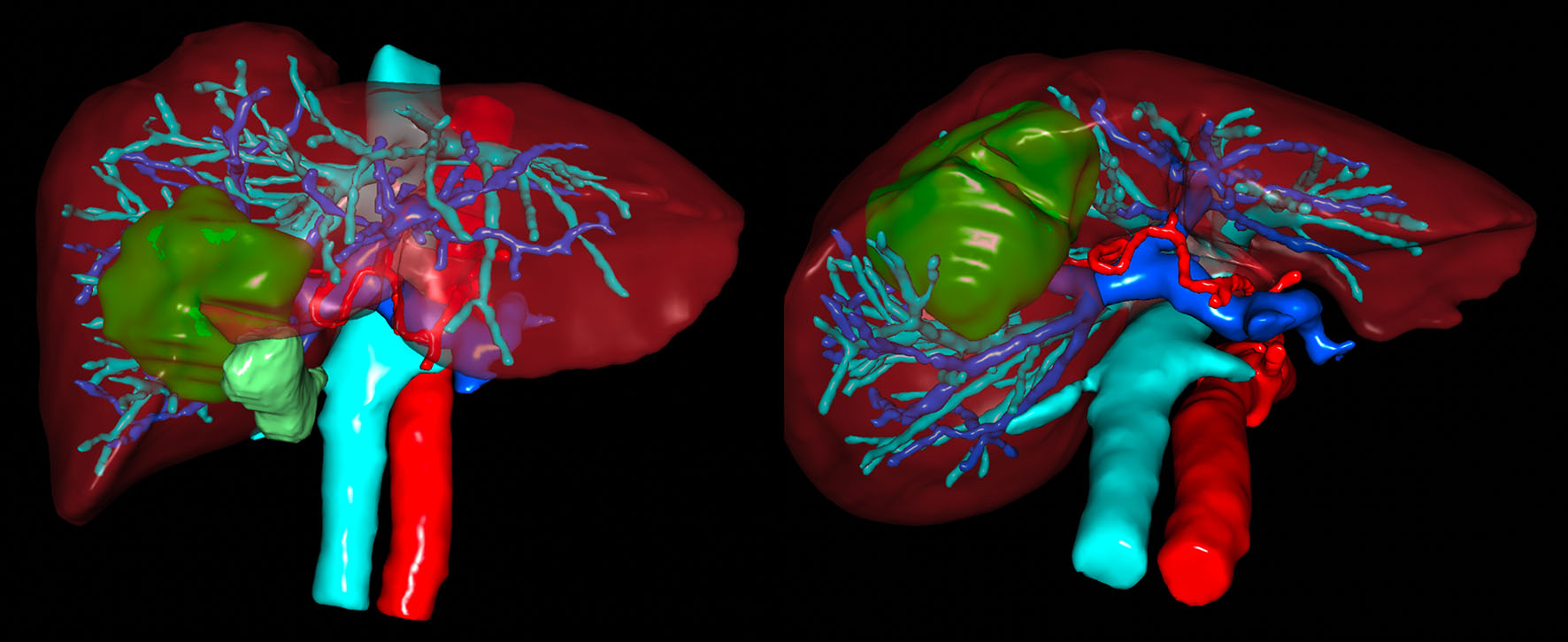
Diagnosis is based on typical imaging findings from dynamic-magnetic resonance imaging (MRI) and dynamic-computed tomography (CT), as widely described in clinical practice guidelines[3]. The selection of adequate candidates for surgical resection is based on the Child-Pugh classification[4,5]. Preoperative imaging with 3D routine reconstruction is essential to know the presence of anatomical variations [Figure 1]. The IWATE scoring criteria are used to assess the difficulty level preoperatively by considering tumor location and size, the proximity to major vessels, and the fibrosis severity[6].
Figure 1. Example of preoperative 3D imaging with reconstruction of arterial, portal, and venous system and their relationships with the tumor. 3D: Three-dimensional.
Major exclusion criteria for minimally invasive approach are: the presence of thrombosis of the portal vein/hepatic vein requiring thrombectomy, and multiple previous laparotomies. Specific exclusion criteria for the down-to-up approach are HCC lesions close to the hepatic-caval confluence. For those cases, our preferred technique is the cranial-to-caudal one following the hepatic veins.
Patient positioning and port placement
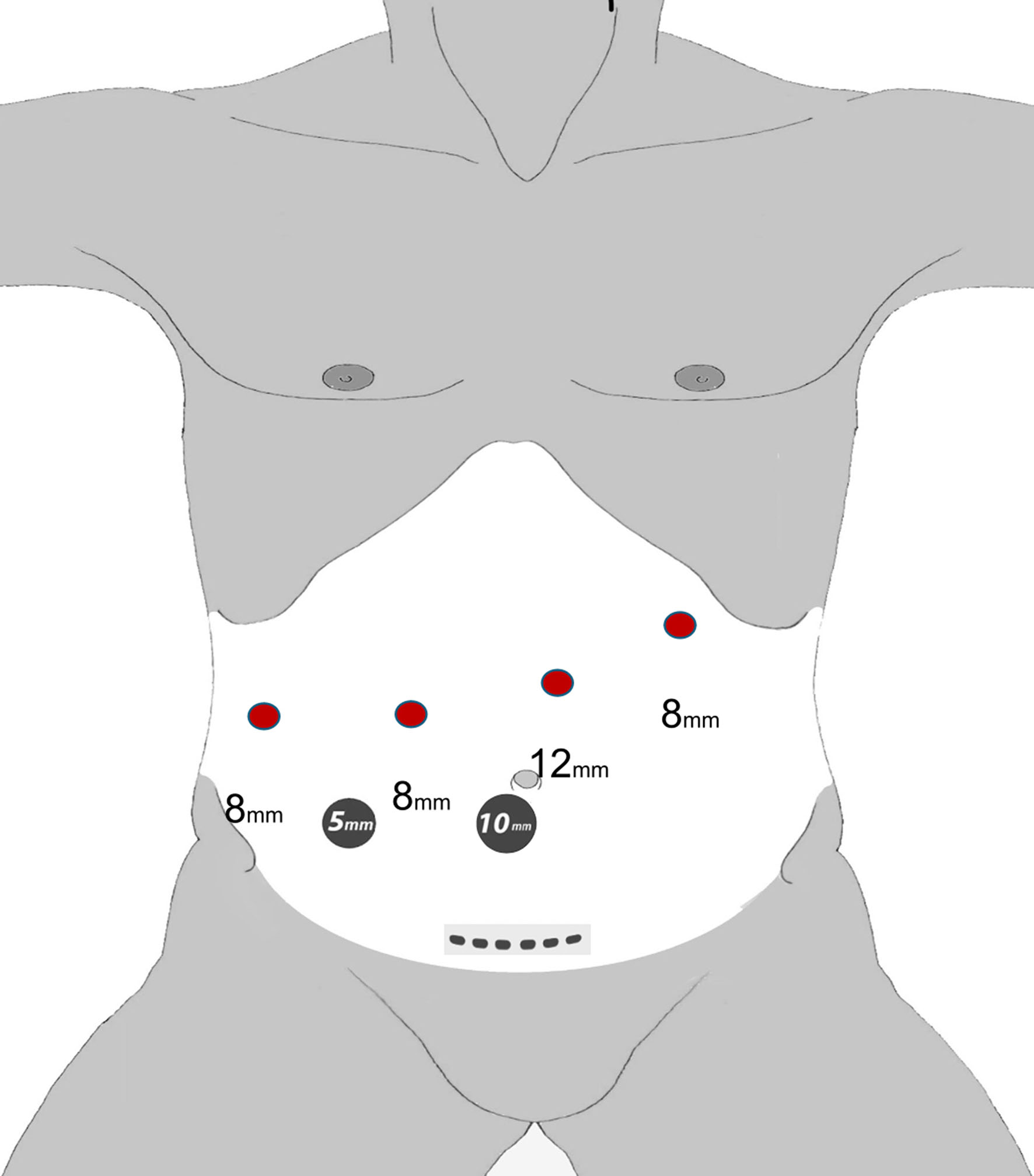
The Da Vinci Xi system (Intuitive Surgical, Inc, Sunnyvale, CA, USA) is used. The French supine position with 15 degrees reverse Trendelenburg is adopted for all robotic hepatic resection. In the case of posterior segment lesions (segments VII and VIII), a cylindrical pillow is positioned along the right side of the patient under the right shoulder and the operating table is tilted toward the left side of the patient, 5 to 10 degrees according to the size of right liver, to facilitate liver mobilization. The arms are fixed at the sides of the trunk. The surgical assistant is positioned in between the open legs of the patient. Pneumoperitoneum is induced by a Veress needle via percutaneous puncture 2 to 4 cm from the edge of left hypochondrium ribs at the level of hemi-clavear point. Once a pneumoperitoneum of 14 mmHg is achieved, the robotic camera is inserted using a 10-12 mm visiport-type laparoscopic trocar at the site of the assisting ports in order to avoid the risk of blind port introduction. Standard port placement is employed with the Xi system docked from the side of the patient. Two assisting ports are used [Figure 2]. The camera is inserted through arm 2 but liberally shifted to arm 3 during the parenchymal liver transection in case the line of transection is not clearly visible from arm 2. Targeting is performed from arm 2.
Robotic instrumentation
Standard robotic instruments are used: two bipolar instruments (a long fenestrated bipolar forceps and the Maryland bipolar forceps), two Cadiere forceps, monopolar curved scissors, a robotic Hem-o-lok applier (size ML and L), and a needle driver.
Laparoscopic instrumentation
The following instruments are used: a long suction device, an advanced bipolar and ultrasonic dissection (ThunderbeatTM Type S, Olympus Medical Systems Corp., Tokyo, Japan), laparoscopic Hem-o-lok applier (size ML, L, XL), and for complex cases, a monopolar electrosurgical electrode (CoolingbisTM, Vecmedical, Montcada i Reixac, Barcelona, Spain). The assistant ports are used for suction, insertion of sutures, sponges, laparoscopic grasper, complementary coagulation, and staplers.
Caudo-peripheral liver parenchymal transection
Firstly, once the pneumoperitoneum is established and the trocars are placed, the hepatoduodenal ligament is exposed and the pars flaccida of the gastrohepatic ligament is opened. The Pringle is achieved by a Foley catheter surrounding the hepatic pedicle for the majority of the cases [Supplementary video 1], with the exception of HCC sited on segments VII and VIII. In these cases, due to the semilateral position of the patient, we prefer an extracorporeal Pringle with a tourniquet passing through a 5mm incision on the right side to facilitate the clamping maneuver. A transient hepatic vascular inflow occlusion with 15 min of clamping followed by 5 min of de-clamping is our reference technique.
Intraoperative ultrasound (IOUS) is realized with the ultrasound probe entered through the 12 mm assistant port by the bedside assistant. The TileProTM function of the daVinciSurgical System® enables the integration of the ultrasound images and the 3D reconstruction into the display during surgery for both the console surgeon and the assistant. After identifying the pedicles by ultrasound and marking the edges of tumoral margins on the hepatic surface, the right or left liver is mobilized according to the localization of the HCC. The caudo-peripheral liver parenchymal transection begins with the isolation of the pedicles to be divided by an extra Glissonean approach. During this step, avoiding forced maneuvers is essential in order to preserve Glissonean capsule, protecting biliary and vascular elements.
Subsequently to the pedicle ligation, the intraoperative administration of 2.5 mg/body of indocyanine green (ICG) is performed according to the negative staining technique: the near-infrared (NIR) light camera enables clear identification of the portal territory of interest, while the rest of the liver fluoresces green [Figure 3]. The Da Vinci’s Firefly modeTM represents another strength of the robotic system in identification of key landmarks. This integrated NIR fluorescence imaging function is activated directly by the surgeon at the console.
Figure 3. Indocyanine green dye negative staining technique during anatomical hepatectomy to clearly identify the portal territory of clamped pedicles using the Da Vinci’s Firefly modeTM.
Once the demarcation line of the portal territory has been clearly identified and marked by robotic monopolar scissors, the liver parenchymal transection can be started. This step begins with the incision of the Glisson capsule performed by laparoscopic ultrasonic dissection, penetrating the parenchyma by 1-2 cm. Then, transection is achieved using a Kelly clamp crush technique: the Maryland bipolar forceps on the 3rd arm dissects the liver from the pedicles with small bites, rapidly opening and closing the jaws. Complementary hemostasis is performed by diathermy with the bipolar forceps on the 1st arm for vessels up to 5 mm. In this phase, the role of the assistant is to follow the robotic instruments with quick irrigation and aspiration in order to always guarantee optimal functioning of the bipolar forceps.
Larger caliber vessels are dissected and surrounded at 360° so as to be able to apply the medium-large Hem-o-lok by the bedside surgeon when the maneuver is laparoscopically easy, otherwise using the robotic applier. A trick we use in the most challenging cases is to load the vessel on a Vicryl suture in order to suspend and expose it as best as possible, ensuring it is completely surrounded before cutting. The suspension of the vascular elements on a tape is a useful measure in robotic surgery because it allows indirect traction of delicate structures without tearing them due to the lack of tactile feedback.
When feasible and easy for the assistant, the sectioning of coagulated and clipped vessels is performed laparoscopically to save time otherwise spent on changing robotic instruments. The liver parenchyma is dissected from the caudal to the cranial aspects of the liver following the pedicles.
Supplementary videos 2-4 extensively show the caudal-to-cranial technique combined with the robotic Maryland Kelly clamp crushing parenchymal transection applied to anterior [Supplementary video 2], posterior [Supplementary video 3] segments resections, and major hepatectomies [Supplementary video 4], respectively.
Intraoperative complications management
With the advent of minimally invasive approaches, bleeding control has become one of the most important criteria for establishing not only the safety and feasibility of the technique but also the surgeon’s proficiency, and constitutes the most frequent reason for conversion to laparotomy. Moreover, intraoperative blood loss is an independent predictor of recurrence and survival after HCC surgery. Tumor size, location, and the extent of resection represent important risk factors for vascular injuries. Intraoperative bleeding control in hepatobiliary surgery is multimodal. The first step is communication with nurses and anesthesia team. In this phase, it is essential to decrease tidal volumes and positive end-expiratory pressure (PEEP) in order to reduce central venous pressure and, consequently, hepatic venous back-bleeding. Close monitoring of the patient is also necessary to identify the possibility of a CO2 embolism as early as possible; in this case, conversion to laparotomy is mandatory. From a purely technical surgical point of view, we essentially follow three steps: control, exposure, and repair. The first thing to ensure is the control of bleeding, which is achieved by using the Pringle maneuver, clamps or temporary clips, and hemostatic gauze (SURGICELTM Absorbable Hemostat). Once the hemorrhage has been controlled, the structure of origin is clearly identified, and the surgeon can dissect it above and below, paying attention to not worsening the injury. In this phase, the bedside assistant plays a crucial role in ensuring correct vision through aspiration and irrigation of the operating field. The optimal exposure is essential to choose the correct repair strategy beyond suturing or stapling, clipping, or using advanced laparoscopic energy devices such as saline-coupled bipolar sealing device (Aquamantys®) or monopolar electrosurgical sealing dissector (Coolingbis®).
Another important precaution is to detect an intraoperative bile leak (IOBL) and properly managing it is essential to prevent postoperative biliary fistula. Bile leak check is performed with a white gauze and any bile leakage is closed with a 5/0 absorbable monofilament. According to our experience, the key to achieving good results in intraoperative complications management is to be ultra-selective in hemostasis and biliostasis, using sutures or bipolar diathermy and avoiding monopolar coagulation which, due to energy transmission, could itself cause iatrogenic ischemic lesions in the surrounding parenchyma.
Specimen extraction, hemostasis, and drains policy
The specimen is placed in an endobag and extracted through an 8- to 10-cm Pfannenstiel incision. Hemostasis is checked carefully once the Pringle maneuver is released, and whenever necessary, one or more TachoSil(®), a medicated sponge coated with human fibrinogen and human thrombin, are applied on the liver surface. One multi-lamellar drain is positioned in the right hypochondrium in case of major hepatectomy or doubtful bile leak test.
TOWARD AND BEYOND THE PILLARS OF HERCULES: HOW THE ARTIFICIAL INTELLIGENCE (AI) IMPACTS MINIMALLY INVASIVE LIVER RESECTIONS AND THE “PRECISION SURGERY” CONCEPT
3D printing of livers with cancers: patient education and surgical planning
One of the emerging applications of AI is patient education. This is a critical component of healthcare, as it enables people to understand their own medical diagnosis, treatment options, and preventive measures. It is well known that a patient who is aware of his illness and well informed about therapeutic prospects is also much more compliant with the treatments offered to him, resulting in better health outcomes. In this context, several AI-based chat boxes are emerging with the purpose of educating and providing information to patients and their caregivers on dietary recommendations and lifestyle modifications such as smoking cessation and behavioral therapies[7]. From a purely surgical point of view, a huge advantage that we found in the use of 3D reconstructions was that of being able to inform patients in detail about the size and location of the neoplasm and perform a simulation of the resection together with them, showing which part and how much of liver will remain after surgery. This illustration is helpful in further understanding possible complications and their management.
The most recent deep learning models can accurately predict surgical risk (i.e., intraoperative blood loss and complications stratified according to Clavien-Dindo classification) based on arterial preoperative CT imaging phases and other clinical data, including age, sex, body mass index, preoperative American Society of Anesthesiologists physical status classification, the occurrence of diabetes mellitus, Child-Pugh classification status, platelet count, and surgical approach[8].
This tool therefore represents a real communication platform, and in our hospital, it is shared not only with patients but, by adding more technical details, also with students and residents who can benefit from their training and comprehension of the surgical procedures they will witness.
AI through ICG, simulation, navigation, and radiomics for HCC
AI technology is also used for augmented reality (AR) navigation, enabling surgeons to overcome the limits of already known systems. The application of AR provides a semi-transparent overlay of the preoperative images on the working area. Liver neoplasm, vascular and biliary structures reconstructed using preoperative CT are projected on the hepatic surface during parenchymal transection, giving the possibility of a simulation before reaching the point of no return and resulting in more accurate and safer resections[8].
Research in the field of medical imaging has focused heavily on AI-based radiomics.
Radiomics is a new discipline that works by extrapolating many features from radiological images, which are then processed using appropriate data analysis methods. This not only enables more accurate diagnoses but also provides valuable information on specific tumor characteristics, such as predicting the response to a treatment, or highlighting the presence of particular genetic and epigenetic alterations, which could be obtained only by using other biomedical technologies. The application of AI in radiomics has significantly improved the precision and efficiency of ultrasound diagnosis, including intraoperative ultrasonography[9].
Another routine technique is ICG fluorescence imaging, which has high sensitivity (> 90%) in tumor and resection margin detection. However, a relatively high number of false positives may represent a limitation of this technique. The integration of preoperative images into the intraoperative field provided by AI increases the fluorescence imaging precision[10]. The most sophisticated models release even a virtual biliary tree depiction.
In our daily practice, the application of 3D reconstructions and AR navigation has proven to be very useful to improve the precision in liver resections and to facilitate communication between the main operator, the bedside assistant, and young surgeons who attend the operation from the outside.
CONCLUSION
To be defined as safe and oncologically correct, a liver resection must be radical to avoid recurrences while also respecting the anatomy and being tailored to save as much healthy parenchyma as possible, thereby minimizing the risk of postoperative liver failure. This is especially true for HCC hepatectomies, where anatomical resection is mandatory and patients often suffer from altered liver function. The overall advancement of minimally invasive techniques and perioperative management has improved the surgical outcomes in cases selected according to the Barcelona Clinic Liver Cancer operability criteria[11].
The most recent recommendations regarding the robotic approach in liver resections come from the 2023 International Consensus Statement on robotic hepatectomy. Based on the latest literature, robotic liver resections seem to be associated with less intraoperative blood loss, shorter hospital stay, and lower postoperative pain. Concerning robotic approach for HCC, it is safe and feasible with lower overall complication rates and similar oncological outcomes despite the longer operative time compared with laparoscopic and open approaches[12].
The main Achilles’ heel of the DaVinci system in liver surgery remains the parenchymal transection due to a lack of standardization and, above all, a limited robotic armamentarium. All robotic liver transection techniques proposed so far, including the present, are under development in the IDEAL (Idea, Development, Exploration, Assessment and Long-Term Follow-Up Collaboration) evaluation system[13]. Their improvement and systematic adoption in selected cases will enable future validation of the results in both the short and long term.
The study group of precision anatomy for minimally invasive hepato-biliary-pancreatic surgery (PAM-HBP surgery)[14] defined the hepatic vein-guided approach (HVGA) using the Glissonean approach as safe and useful in minimally invasive anatomic liver resections, as these veins are considered the most important landmarks for the division of the liver parenchyma. Moreover, it distinguished three different approaches of vein guided transection (cranio-ventral, cranio-dorsal, and caudo-peripheral approach). Currently, there is a lack of studies in the literature with an adequate sample of patients demonstrating the superiority of one approach over the other. In a recent paper including a total of 36 patients, Kawasaki et al. compared the cranio-dorsal and the caudo-peripheral technique for laparoscopic left lateral sectionectomy, describing the first as the safest approach because, in this case, transection is performed from the root side to the peripheral side, moving the tips of CUSA with a back-scoring motion which can avoid injury of the branches when exposing the hepatic vein[15]. However, based on our experience, these principles valid for laparoscopy cannot be entirely applied to the robotic approach. This is because parenchymal transection tools differ, and the CUSA in our case is replaced by the Maryland bipolar with a finer and more delicate dissection movement assured by the Da Vinci platform. Furthermore, in cases of large tumors, it could be complicated to overturn the left lobe and this gives a limited view of the operating field when using the caudo-dorsal approach.
In our practice, the integration of the caudo-peripheral technique and the extra Glissonian approach to the pedicles, already widely adopted in our laparoscopic practice, has enabled us to take full advantage of the DaVinci system, including the 3D magnification of the visual field and the flexibility and tremor filtering capabilities of robotic arms. Since in cirrhotic liver, the pedicle’s looping and isolation are more challenging due to the fibrosis and bleeding susceptibility, robotic wristed instruments can facilitate the pedicle encirclement maneuvers but also suture in cases of unexpected hemorrhage[16].
The section of the pedicle allows on one side to have a clear line of demarcation ensured by the ICG negative staining technique and on the other to proceed with a safe parenchymal transection with optimal bleeding control guaranteed on several fronts: the Maryland bipolar Kelly clamp crushing technique, the robotic bipolar forceps on the other arm, the intermittent Pringle’s maneuver, and in cases of need for complementary hemostasis, intervention of the bedside assistant with Hem-o-lok or an advanced laparoscopic bipolar instrument. The assistant also has the role of ensuring correct and clear vision by performing laparoscopic irrigation and suction.
Multi-pronged bleeding control allows for faster progress in the transection procedure and consequently reduces the overall number of intermittent clamping times. This is a key point to minimize the risk of ischemia-reperfusion injury and liver congestion.
Adopting a technique already performed by laparoscopy certainly makes the learning curve of the same skill by robotic approach faster. However, unlike other types of surgery that can benefit from a fully robotic approach, liver resections require the active collaboration of the bedside assistant, who must have extensive experience in laparoscopic hepatobiliary surgery. Our technique does not go beyond this limit. During the procedure, the assistant must use one or more laparoscopic dissection and coagulation instruments, and apply Hem-o-lok or stapler, which demands perfect synchronization with the surgeon operating at the console, along with the ability to effectively overcome the limitations inherent in laparoscopy, such as reduced flexibility and, in some cases, limited access to the dissection plane.
Advances in AI technologies have shown great potential to reshape the management of clinical care, preoperative planning, and intraoperative decision making for patients with HCC. The application of 3D reconstructions for surgical planning and simulation, AR navigation, and AI-based radiomics have strengthened the concept of “precision surgery” and established an optimal communication platform for patient education and information transmission between surgeons. However, the lack of standardized algorithms and software, the inability to explain AI statements due to the “black box” phenomenon, and the “AI chasm” that means the gap between what is expected and what is achievable represent, for the moment, the greatest limit to the diffusion of AI across the institution.
The lack of a qualified team makes the surgical procedures described so far unsafe and unfeasible. Technology is crucial but does not replace surgical experience.
DECLARATIONS
Authors’ contributions
Conceptualization: Rosso E, Fassari A
Methodology: De Blasi V, Rosso E, Fassari A
Validation: Rosso E, Ielpo B, Anselmo A
Technical and material support: Rosso E, De Blasi V, Dalla Valle B
Writing - original draft preparation: Fassari A, Rosso E
Writing - review and editing: Fassari A, Rosso E, Ielpo B, Anselmo A
Visualization: De Blasi V, Dalla Valle B
Supervision: Rosso E
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The Ethical Board confirmed that ethical approval was not required for this technical note.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
1. Otsuka Y, Kaneko H, Cleary SP, Buell JF, Cai X, Wakabayashi G. What is the best technique in parenchymal transection in laparoscopic liver resection? Comprehensive review for the clinical question on the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2015;22:363-70.
2. Navinés-López J, Pardo Aranda F, Cremades Pérez M, Espin Álvarez F, Zárate Pinedo A, Cugat Andorrà E. Microfracture-coagulation for the real robotic liver parenchymal transection. J Robot Surg. 2024;18:101.
3. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
5. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-9.
6. Tanaka S, Kawaguchi Y, Kubo S, et al. Validation of index-based IWATE criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery. 2019;165:731-40.
7. Xu FWX, Tang SS, Soh HN, Pang NQ, Bonney GK. Augmenting care in hepatocellular carcinoma with artificial intelligence. Art Int Surg. 2023;3:48-63.
8. Shinkawa H, Ishizawa T. Artificial intelligence-based technology for enhancing the quality of simulation, navigation, and outcome prediction for hepatectomy. Art Int Surg. 2023;3:69-79.
9. Grewal M, Ahmed T, Javed AA. Current state of radiomics in hepatobiliary and pancreatic malignancies. Art Int Surg. 2023;3:217-32.
10. Ishizawa T. “Bon mariage” of artificial intelligence and intraoperative fluorescence imaging for safer surgery. Art Int Surg. 2023;3:163-5.
11. Brozzetti S, D’Alterio C, Bini S, et al. Surgical resection is superior to TACE in the treatment of HCC in a well selected cohort of BCLC-B elderly patients-a retrospective observational study. Cancers. 2022;14:4422.
12. Liu R, Abu Hilal M, Wakabayashi G, et al. International experts consensus guidelines on robotic liver resection in 2023. World J Gastroenterol. 2023;29:4815-30.
13. Di Benedetto F, Petrowsky H, Magistri P, Halazun KJ. Robotic liver resection: hurdles and beyond. Int J Surg. 2020;82S:155-62.
14. Gotohda N, Cherqui D, Geller DA, et al. Expert Consensus Guidelines: how to safely perform minimally invasive anatomic liver resection. J Hepatobiliary Pancreat Sci. 2022;29:16-32.
15. Kawasaki Y, Yamasaki Y, Idichi T, et al. Usefulness of cranio-dorsal approach for laparoscopic left lateral sectionectomy. Updates Surg. 2023;75:889-95.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.




















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].