ENose: a new frontier for non-invasive cancer detection and monitoring
Abstract
Electronic Nose (ENose) technology has emerged as a transformative tool in medical diagnostics, leveraging sensor arrays that mimic the human olfactory system to detect odors and volatile organic compounds (VOCs) in various biological samples. ENose systems utilize a range of sensor types, such as metal oxide semiconductors and conducting polymers, to generate unique “smell fingerprints” through pattern recognition algorithms. These systems have shown promise in diagnosing various medical conditions, including respiratory diseases, infectious diseases, metabolic disorders, and neurological conditions. Notably, ENose technology holds significant promise in cancer diagnostics, offering a non-invasive, cost-effective, and rapid approach to early detection and monitoring. It has demonstrated impressive accuracy (85%-95%) in detecting cancers and monitoring complications. However, challenges remain, including issues with standardization, sensor sensitivity, and data interpretation. Despite these hurdles, ENose technology’s market growth is fueled by the increasing prevalence of chronic diseases. Recent developments in Artificial Intelligence (AI), particularly machine learning techniques like deep learning, have enhanced the diagnostic accuracy and robustness of ENose devices. This paper explores the evolution, core principles, applications, challenges, and future potential of ENose technology, with particular emphasis on integrating recent advancements in AI for enhanced detection and interpretation. Future research and collaboration across sectors are essential to overcome existing challenges and integrate ENose into mainstream healthcare.
Keywords
INTRODUCTION
Electronic Nose (ENose) technology is an intriguing topic that emulates the sense of smell in electronic gadgets. Like the human olfactory system, these technologies detect and recognize odors or volatile chemicals. ENose systems operate based on pattern recognition. They are made up of a series of sensors that detect various volatile substances in the air. Each sensor responds uniquely to different odors, resulting in a distinct “smell fingerprint” for each molecule[1]. When exposed to a sample, the sensors emit signals, which are then processed and analyzed to identify and categorize the odor based on its distinct pattern. An ENose is often made up of a sensor array comprising a variety of sensors, including Metal Oxide Sensors (MOS) and conducting polymers. These sensors sense volatile chemicals and send signals. A data collection system captures and processes these signals, while pattern recognition algorithms use the data to detect and categorize odors. The data are then shown through an output interface, giving real-time feedback on odor detection[2-4].
In this review, we aimed to give an in-depth assessment of ENose technology, with an emphasis on its current and future applications for cancer diagnostics. We explored the underlying concepts of ENose technology, charting its evolution from early industrial prototypes to its critical role in medical diagnostics, focusing on the detection of disorders via breath Volatile Organic Compounds (VOCs). The article emphasizes the benefits of ENose in oncology, including early cancer identification, increased patient comfort, faster findings for timely therapies, preventative tactics, lower risk of complications, affordability, and remote monitoring of patients. Furthermore, new trends and future directions in ENose technology are described, highlighting the ability for integration into wearable devices, applications in precision oncology, and greater clinical acceptability, as well as the existing hurdles and prospects for additional study.
The remainder of the paper is structured as follows: After the introduction, Section 1 provides a comprehensive overview of the history and development of ENose technology in medical applications. Section 2 highlights the importance of rapid, non-invasive diagnostic tools in healthcare and explores the principles of ENose technology, focusing on sensor arrays and detection mechanisms. Section 3 delves into the applications of ENose in cancer diagnostics, examining its role in detecting various cancers, including lung, breast, gastrointestinal, prostate, head and neck, and bladder cancers. Section 4 addresses the challenges associated with ENose technology in medical diagnostics. Section 5 explores future directions and opportunities, including ENose’s potential in remote cancer monitoring and telemedicine, the significance of collaborative and interdisciplinary research, and its clinical implementation. Finally, the paper concludes in section 5.
The history of ENose in medical applications and cancer detection
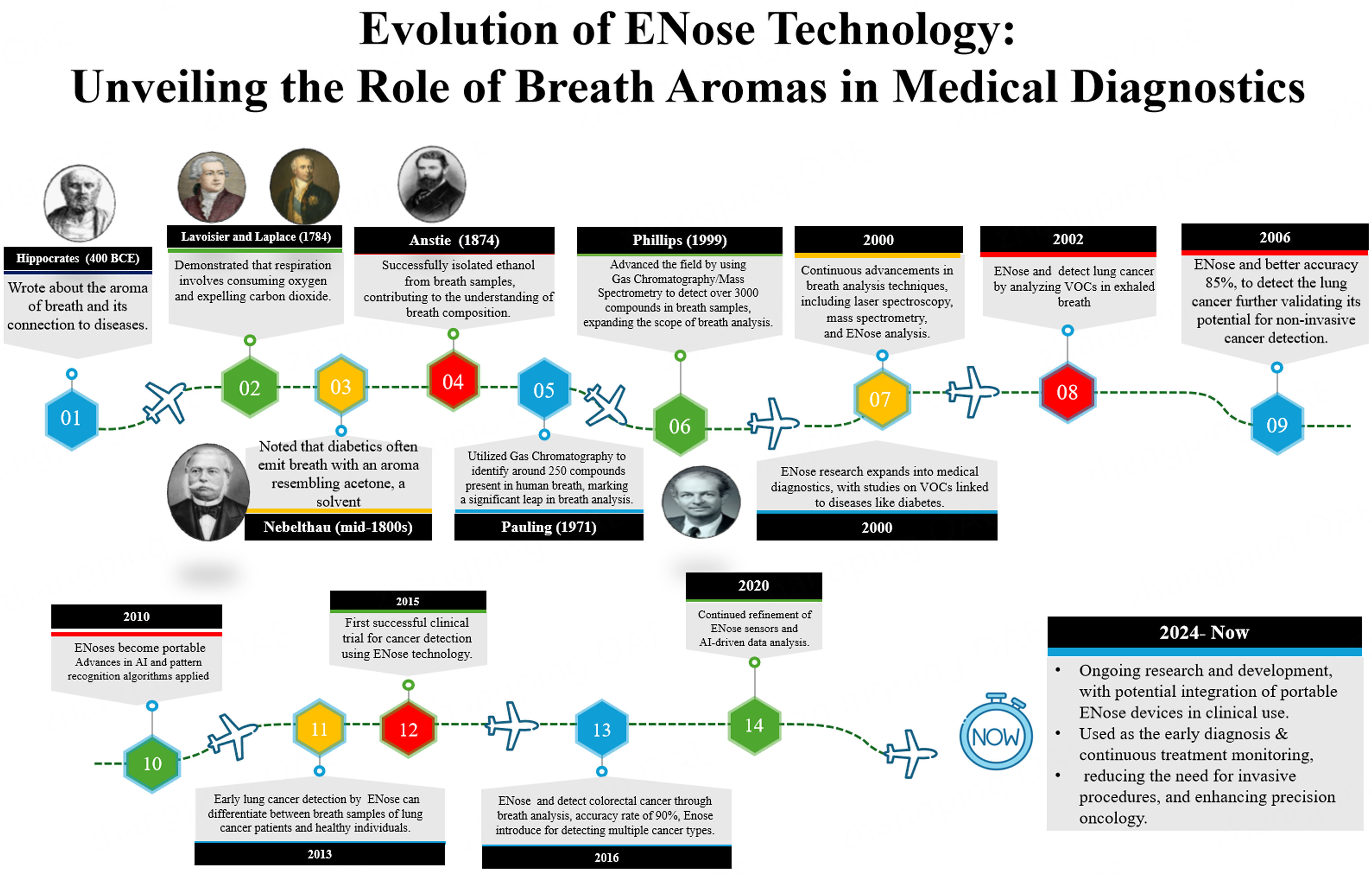
The history of ENose technology in medicine, particularly in cancer detection, reflects a journey of innovation, from its early conceptualization to its current integration of Artificial Intelligence (AI)-driven advancements in clinical applications. Originally developed in the late 20th century for industrial and environmental purposes, ENose technology began to transition into medical research in the 1990s, with a focus on disease diagnosis through the detection of VOCs in breath [Figure 1]. During this period, the development of sensor arrays using metal oxide semiconductors and conducting polymers initiated the exploration of using ENose for detecting diseases via breath VOCs[5]. By the 2000s, advancements in sensor technology and algorithms significantly enhanced the accuracy of ENose devices. This period saw the first clinical trials applying ENose technology to detect various illnesses, including lung cancer and diabetes. The use of breath analysis to identify VOCs linked to cancer began to show real promise, particularly for lung cancer detection[6]. A significant milestone occurred in 2002 when researchers demonstrated that
Figure 1. History of ENose in Medicines with respect to breath aromas and cancer. ENose: Electronic nose.
In 2013, researchers at the University of Manchester demonstrated the potential of ENose technology to differentiate between breath samples from lung cancer patients and healthy controls, offering promising results for early lung cancer detection (van de Goor et al., 2018). This study marked another important step in validating ENose as a tool for cancer diagnosis[10]. A breakthrough occurred in 2015, when clinical trials using ENose technology for cancer detection, specifically lung cancer, demonstrated successful results by analyzing VOCs in exhaled breath. This success paved the way for further exploration of ENose technology in early cancer detection, with a particular emphasis on non-invasive diagnostic methods[11]. Another major milestone was achieved in 2016 by researchers at the University of California, Berkeley, who used an ENose to detect colorectal cancer through breath analysis. The ENose was able to identify cancer-specific VOCs with an impressive accuracy rate of 90%, further broadening the scope of ENose applications in cancer diagnostics[12]. As we move into the 2020s, ENose technology continues to evolve with further improvements in sensors and enhanced AI algorithms that drive more accurate data interpretation. Its integration into clinical settings is expanding, not only for cancer detection but also for monitoring treatment effectiveness and detecting recurrence, particularly in cancers such as colorectal and gastric cancers[13]. Looking ahead, 2024 marks an exciting milestone as portable ENose devices are being developed for real-time, non-invasive cancer monitoring. These advancements are enhancing precision oncology by enabling early diagnosis and continuous monitoring of treatment responses, thereby improving patient outcomes while reducing the need for invasive tests. Ongoing research and development in this field hold the potential to revolutionize cancer care by providing early detection and more effective disease management strategies[14].
IMPORTANCE OF RAPID AND NON-INVASIVE DIAGNOSTIC TOOLS IN HEALTHCARE
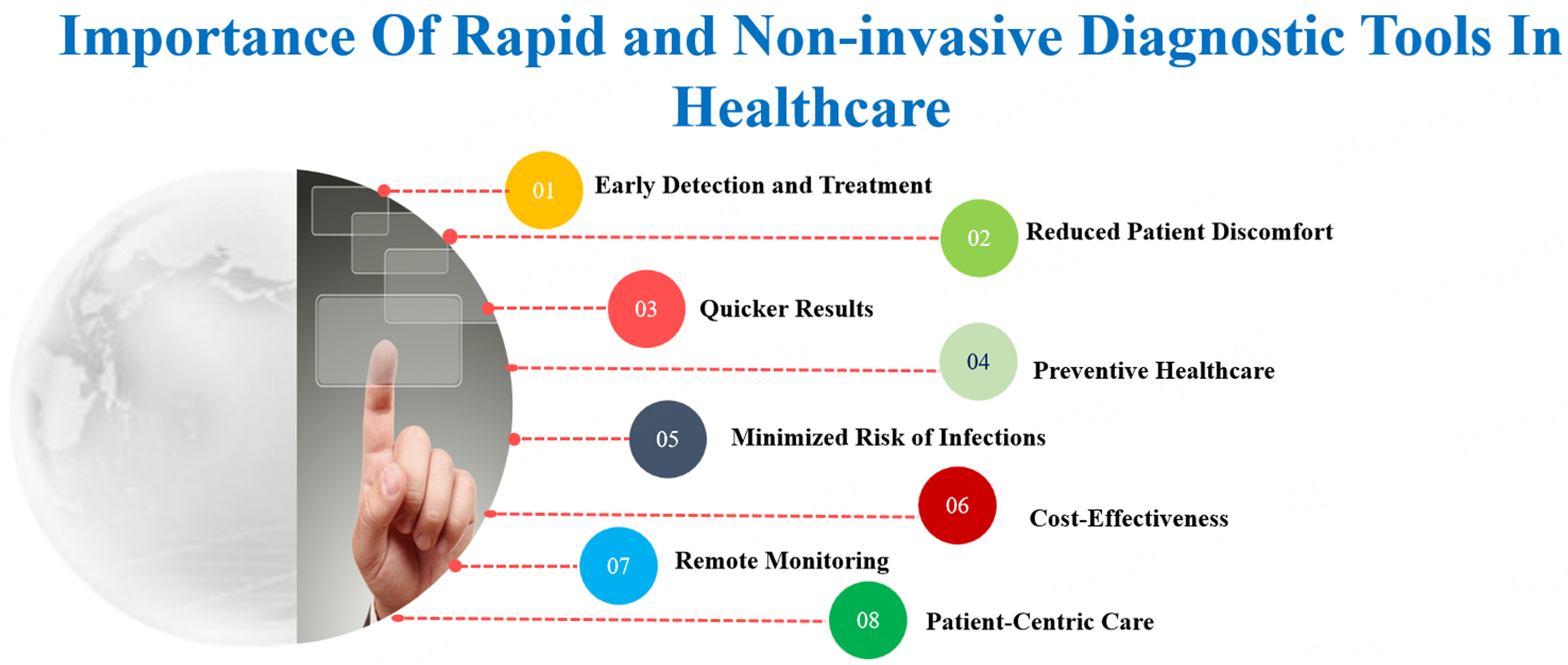
Rapid and non-invasive diagnostic tools play a crucial role in healthcare for several reasons. They facilitate the early detection of diseases, enabling prompt interventions and treatments that enhance patient outcomes while reducing healthcare expenses. Non-invasive tools eliminate the need for invasive procedures or sample collection methods, improving the overall patient experience and encouraging adherence to healthcare protocols[15]. This seems particularly evident in cancer diagnostics, since a prompt intervention can dramatically impact patient outcomes. The rapid and non-invasive diagnostic tools that can be used in healthcare are shown in Figure 2.
Rapid diagnostic tools deliver quick results, aiding healthcare providers in making informed and timely decisions about patient care, particularly relevant in oncology, which is characterized by time-sensitive treatments. These tools bolster preventive healthcare efforts by enabling early detection, identifying risk factors, and preventing disease progression or complications. Non-invasive diagnostic tools lower the risk of healthcare-associated infections (HAIs) from invasive procedures, enhancing patient safety and reducing strain on healthcare systems. Most often, they serve as screening tests and only when positive, followed by more accurate analysis; this makes them available for large fractions of patients in a time a cost-effective way. In fact, financial savings result from reducing the need for multiple tests, hospital stays, and invasive procedures, making healthcare more efficient and accessible. Remote patient monitoring using non-invasive tools allows healthcare providers to track disease progression, assess treatment efficacy, and intervene as needed without frequent in-person visits. Patients benefit from quick access to diagnostic information, which promotes active participation in healthcare decisions and enhances overall engagement with healthcare services[16-18].
Principles of ENose technology
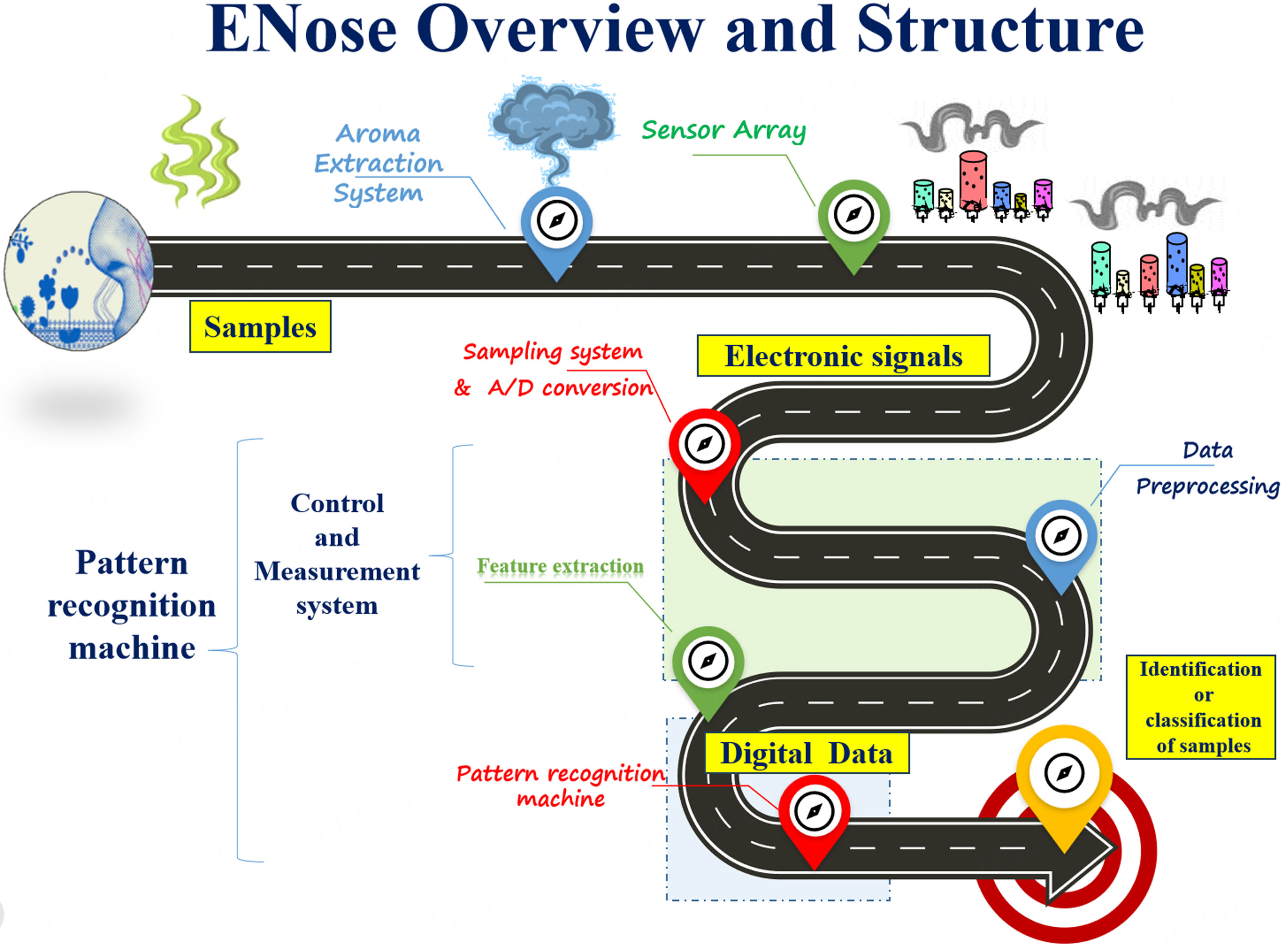
ENose technology operates on several underlying principles and utilizes a sensor array that responds to VOCs or odors, generating unique electrical signals known as “smell fingerprints”[19]. These signals are processed through pattern recognition algorithms, often employing machine learning techniques for odor identification. Calibration ensures accurate odor recognition, with results presented through an output interface for real-time feedback, enabling applications in medical diagnostics, environmental monitoring, and security, as shown in Figure 3.
Briefly, ENose works through these different steps[20]:
Sensor array: An ENose typically consists of an array of chemical sensors, each designed to respond to different VOCs or odors. These sensors may include MOS, conducting polymers, Quartz Crystal Microbalances (QCM), and Surface Acoustic Wave (SAW) sensors.
Pattern recognition: When exposed to a sample containing VOCs, the sensors generate electrical signals based on their interactions with the compounds. The pattern of these signals, often referred to as the “smell fingerprint”, is unique for each VOC.
Data acquisition: The signals from the sensor array are collected using a data acquisition system. This system digitizes the analog signals for further processing.
Pattern recognition algorithms: Advanced algorithms process digitized sensor data to identify patterns and distinguish between different odors. Machine learning techniques, such as neural networks or support vector machines, are commonly used for this purpose.
Calibration: ENose requires calibration to establish baseline responses for various odors. This calibration ensures accurate and consistent odor recognition over time.
Response analysis: The processed data are analyzed to identify and classify the detected odor based on its unique pattern. This analysis may involve comparing the sensor response pattern to a database of known odor patterns or using statistical methods to determine similarity scores.
Output interface: The results of the odor analysis are typically presented through an output interface, such as a graphical display or numerical output. This interface provides real-time feedback on the detected odor and its concentration or classification.
By combining sensor technology, pattern recognition algorithms, and data analysis techniques, ENose technology can effectively mimic the human olfactory system and detect a wide range of odors or VOCs in various applications, including medical diagnostics, environmental monitoring, quality control, and security[21].
Sensor arrays and detection mechanisms
ENose sensor arrays contain a variety of sensor types, including MOS, conducting polymers, QCM, SAW sensors, optical sensors, and capacitive sensors. These sensors detect VOCs via a variety of processes, including changes in electrical conductivity, mass shifts on quartz crystals, changes in acoustic wave propagation, differences in optical characteristics, and capacitance changes. The combined reactions of these sensors provide a unique “smell fingerprint”, which is analyzed using pattern recognition algorithms to identify and categorize odors, improving sensitivity and selectivity in odor identification. These sensors’ detection processes use physical or chemical interactions between VOCs and sensor surfaces, which cause detectable changes in electrical, acoustic, optical, and mechanical characteristics.
The sensor array’s combined reaction to numerous VOCs creates this “smell fingerprint”, which pattern recognition algorithms use to reliably detect and categorize odors. Integrating several sensor types into an array enhances the sensitivity, selectivity, and overall capabilities of ENose systems for detecting odors[22-24].
Applications of ENose in cancer diagnostics
ENose technology is used in medical diagnostics to diagnose a wide range of conditions via non-invasive breath analysis, including respiratory diseases, malignancies, infectious agents, and metabolic problems [Figure 4]. It also helps to monitor organ transplants, measure wound healing, identify narcotics and alcohol, and enable personalized medication[25,26]. As far as cancer is concerned, new methods for early-stage detection are under investigation. A few of them, including those using gas chromatography (GC) and mass spectrometry (MS), are based on gaseous sample analysis. These devices are expensive, difficult to use in daily medical practice, and have limited portability, making them only suitable for organized laboratory environments. By contrast, cheaper portable ENose instruments can detect very low amounts of molecules, distinguishing between volatile metabolites in complex mixtures[27].
Figure 4. Various uses of ENose in medical research and Cancer diagnosis. ENose: Electronic nose; VOCs: volatile organic compounds; CT: computed tomography; MRI: magnetic resonance imaging; PET: positron emission tomography.
Therefore, so far, breath analysis is one of the most promising uses for ENose technology. Cancer cells produce distinct metabolic byproducts, which can be identified as particular VOCs in the breath. In addition, urine and perspiration contain VOCs that can be used as cancer biomarkers. The ENose detects these chemicals with excellent sensitivity, adding another non-invasive tool for early cancer detection.
According to the most recent studies, ENose can distinguish with high accuracy between healthy people’s breath patterns and those with cancers such as lung, breast, and colorectal cancer[28,29]. The most frequently analyzed type of cancer is certainly lung cancer; however, different tumors are also considered in studies using ENose technology.
ENose vs. lung cancer
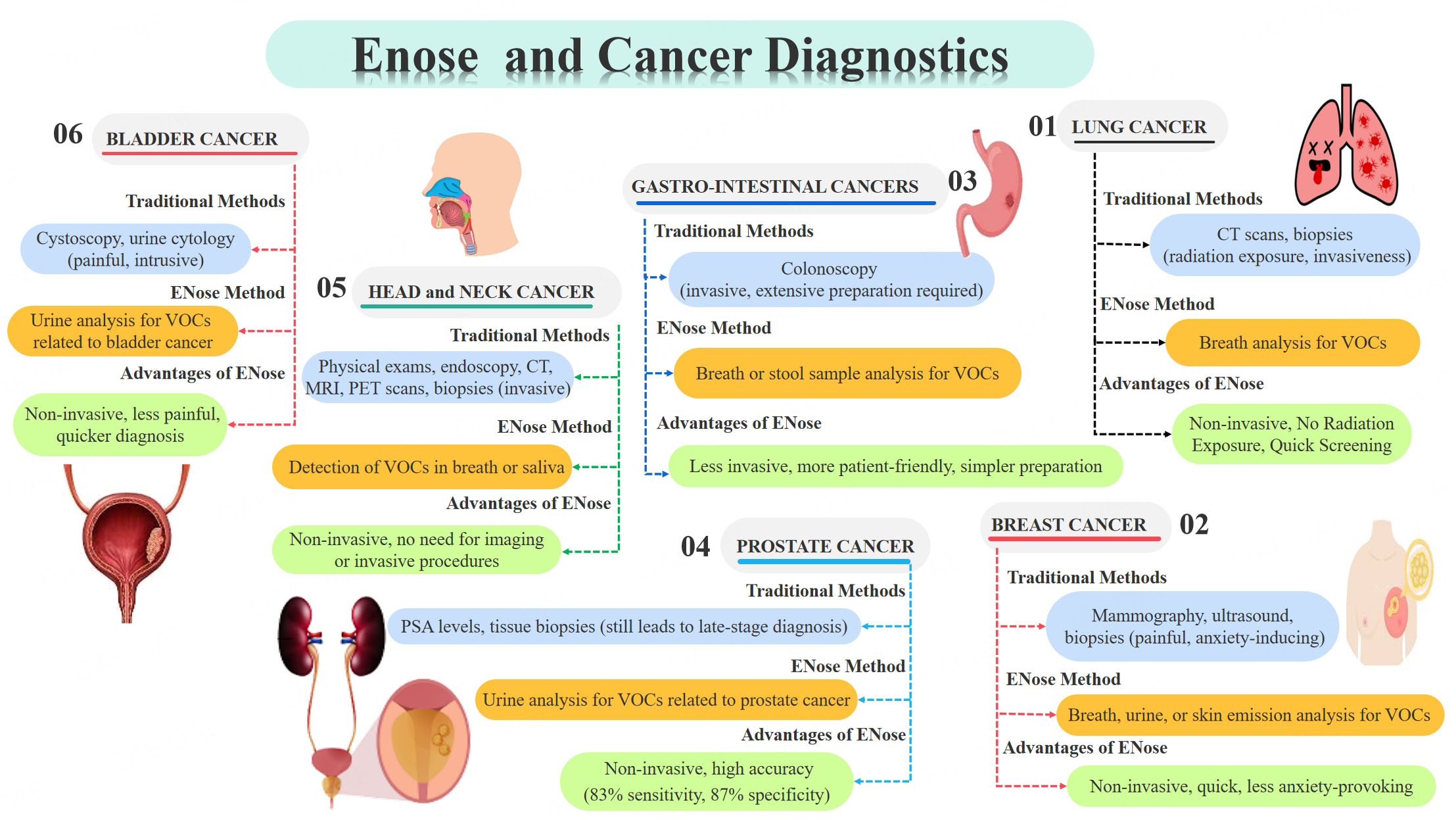
Lung cancer is one of the most common causes of cancer-related death worldwide. Early detection dramatically increases survival rates. Traditional procedures, such as CT scans and biopsies, are only partially successful, and have drawbacks such as radiation exposure and invasiveness[30]. The ENose is a non-invasive alternative that analyzes breath samples for VOCs related to lung cancer. Research has shown that ENose can detect lung cancer with high sensitivity and specificity, making them useful tools for early detection. Most studies using ENose technology achieved satisfactory sensitivity, specificity, and accuracy (An accuracy of over 80% in differentiating between lung cancer (LC) and controls, as well as between LC and other cancer types), despite the low specificity of ENose systems in general. Particularly, a recent study by Rocco et al. indicated an 86% sensitivity and 95% specificity compared to histopathology[31]. In addition, a multicentric study showed an Area Under the Curve (AUC) of 95% when an appropriate AI algorithm was applied[32]. On the other hand, a certain degree of heterogeneity in cancer detection can be due to (1) signal acquisition protocols, (2) the device used, and (3) the studied population (in terms of both size and pre-test disease probability). As far as devices are concerned, the most used nanosensors are either integrated into commercial devices like the Cyranose 320 (Sensigent, Baldwin Park, CA, USA) or as non-commercial products and prototypes. Nanosensors provide numerous advantages over traditional sensors, including fast response times, adequate detection limits, great portability and scalability, good sensitivity and resolution, and high selectivity[33]. Machado et al. published a study in 2005[34] that used a portable chemical vapor detector with 32 composite carbon black-polymer sensors (Cyranose 320) to distinguish between patients affected by lung cancer and controls. VOCs in exhaled air attach to polymers, causing a reversible change in the electrical resistance of sensors. This system showed a sensitivity of 71.4% and a specificity of 91.9%. Di Natale et al.[35] used aniline, alkanes, and benzene derivatives, while D’Amico et al. and Tran et al.[36,37] used aniline, o-toluidine, and cyclopentane, and Peng et al.[38] used isoprene, alkanes, methyl alkanes, and benzene derivatives as disease biomarkers. Remarkably, as technology advanced, nanosensors produced increasingly accurate readings. Bikov et al.[39] found poor performance and difficult airflow control in evaluating VOCs using Principal Component Analysis (PCA) for discriminating between classes, with only 40% specificity in distinguishing between lung cancer patients and healthy smokers. Hubers et al.[40] also found poor specificity in discriminating between patients. The characteristics were reduced using a PCA with six components. PCA performed better in discriminating categories; therefore, it was utilized to create a Receiver Operating Characteristic (ROC) curve to calculate sensitivity and specificity. Sensigent developers have then improved their product’s performance through feedback from trials and technological advancements, as evidenced by large-scale research published by Tirzïte et al. in 2017 and 2019[41,42]. The Cyranose 320 accurately distinguishes between lung cancer patients and controls, as well as different stages of lung cancer, utilizing Support Vector Machine (SVM) and Logistic Regression Analysis (LRA). Future improvements to Cyranose 320 performance should focus on improving specificity and lowering false positives. Huang et al.[43] found that when using Linear Discrimination Analysis (LDA) and SVM for classification, it is important to keep temperature and humidity stable in the testing environment.
MOS sensors have been widely utilized in ENose applications for lung cancer discrimination and continue to be a leading technology in the sector after many years of application. MOS devices offer several advantages, including a wide range of responses, high sensitivity, low cost, fast response time, small size, easy fabrication, long-lasting life, and a wide temperature range. However, controlling these variables is crucial due to their high selectivity, high power consumption, instability, and sensitivity to external changes[44]. Several other studies have followed[45], and overall, the use of ENose systems for lung cancer detection has shown promising results. Numerous studies have demonstrated the effectiveness of ENose technology in detecting the disease, suggesting its potential for broader use, even in clinical practice. However, further technological advancements are necessary to enhance the performance of these systems, along with properly designed diagnostic accuracy studies.
ENose vs. breast cancer
Breast cancer is often detected using mammography, ultrasound, and biopsies. While successful, these procedures can be painful and cause worry in patients[46]. The ENose can detect VOCs in the breath, urine or even skin emissions that are symptomatic of breast cancer, providing a non-invasive and quick screening tool. Early studies have yielded promising findings, indicating that ENoses could supplement established diagnostic approaches, particularly in resource-limited situations. Specifically, when VOCs from urine were assessed, the system achieved an overall accuracy of 75%, with a sensitivity of 100% and a specificity of 50%[47].
ENose vs. gastrointestinal cancers
Colorectal cancer screening frequently relies on colonoscopy, an invasive procedure that necessitates extensive preparation[48]. The ENose analyzes breath or stool samples for VOCs associated with colorectal cancer, providing a less invasive and more patient-friendly screening procedure. Early detection with
ENose vs. prostate cancer
Despite the availability of screening procedures based on Prostate-Specific Antigen (PSA) levels and tissue biopsies, a significant number of patients still present with advanced prostate cancer. Protocols to detect VOCs in the urine of patients affected by prostate cancers have been developed, showing accuracy, sensitivity, and specificity of 83% (CI95% 77-89), 82% (CI95% 73-88), and 87% (CI95% 75-94), respectively[51].
ENose vs. head and neck cancer
The traditional methods for detecting head and neck cancer include physical examination, endoscopy, and imaging tests like Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and Positron Emission Tomography(PET) scans. A biopsy is often performed to confirm the presence of cancer. These methods help assess the size, location, and spread of the tumor[52].
Head and Neck cancer (HNC) has been studied in seven articles, with MOS sensors being the most commonly used due to their widespread and low cost. ENose demonstrated good sensitivity, specificity, and accuracy, comparable to that of LC. For example, van Hooren et al. used ENose to characterize the exhaled breath of HNC patients and differentiated between HNC and colon cancer[53]. Hakim et al. used commercial ENose tools to distinguish between various types of cancer. Two studies utilized nanosensors, with the ENose (NA-NOSE) device showing excellent performance. Hakim et al. achieved 100% sensitivity in discriminating HNC and controls, and 90% sensitivity and specificity in differentiating HNC from lung cancer[54]. Later, Gruber et al. distinguished between HNC and controls and different tumor sites with 90% accuracy[55].
ENose vs. bladder cancer
Bladder cancer is difficult to identify early due to the lack of particular symptoms. Traditional diagnostic procedures, such as cystoscopy and urine cytology, can be painful and intrusive[56]. ENose analyzes urine samples for VOCs linked to bladder cancer. Studies have demonstrated that ENose can distinguish urine samples from bladder cancer patients and healthy individuals with excellent accuracy. Interestingly, studies on bladder cancer also confirmed the importance of the bioinformatic tools accompanying the ENose technology. In fact, Tyagi et al. showed that the sensitivity and specificity of VOC detection in urine were 0.93 and 0.88, respectively, using a Sparse Logistic Regression, and 0.93 and 0.76 using a Random Forest classifier[57]. This confirmed that detection and interpretation must evolve in parallel in order to ensure an accurate diagnosis.
CHALLENGES OF ENose IN MEDICAL DIAGNOSTICS
ENose technology faces various challenges that need to be addressed for its successful implementation in medical diagnostics [Table 1]. One of the major hurdles is calibration and standardization, as ensuring consistent sensor responses across different ENose models is difficult, leading to variations in readings and interpretations[58,59]. Environmental odors further complicate matters, as they can interfere with detecting target odors, reducing the specificity and reliability of the system, especially in complex settings. Another challenge is the complexity of odor profiles, as medical odors often involve a wide range of VOCs in varying quantities, requiring sophisticated algorithms for accurate analysis and interpretation. The sensitivity and selectivity of sensors are critical as ENose sensors need to be finely tuned to detect low concentrations of specific VOCs while remaining selective and not confused by other odors. In addition, validation and clinical utility are key, as more studies are needed to establish the clinical efficacy and predictive value of
ENose challenges and their role in cancer detection
| Challenge | Description |
| Calibration & standardization | Calibration is essential for reliable findings; however, standardizing sensor responses between ENose models is problematic, resulting in inconsistent readings and data interpretation |
| Interference from environmental odors | Environmental odors can disrupt target odor detection, compromising specificity and reliability, especially in complex environments |
| Complexity of odor profiles | Medical odor profiles are complicated with various VOCs and changing quantities, necessitating advanced algorithms for correct analysis and interpretation |
| Sensor sensitivity & selectivity | ENose sensors require optimization to identify small amounts of target substances while staying selective for distinct odors |
| Validation & clinical utility | Validation studies are needed to prove the clinical efficacy, sensitivity, specificity, and prediction value of ENose technology compared to traditional diagnostic procedures |
| Data interpretation and analysis | Skill in analyzing data, pattern identification, and statistical modeling is essential for proper ENose data interpretation and diagnosis |
| Cost and accessibility | Adoption may be hindered by initial expenditures, maintenance, and calibration fees, necessitating efforts to make it affordable and accessible to healthcare professionals and patients |
| Integration with clinical workflow | Successful deployment in medical practice requires seamless integration into clinical processes, user-friendly interfaces, and compliance with EHRs |
| Water vapor interference | Water vapor in breath samples can interfere with ENose readings by masking or diluting VOC signals, complicating accurate disease detection, and reducing diagnostic reliability. Both endogenous and exogenous water contribute to this challenge |
| Biomarker overlap & influence | Some cancer biomarkers may overlap, making it difficult to distinguish between diseases. Additionally, syndromes can influence results, complicating diagnosis and increasing the challenge of accurate detection |
FUTURE DIRECTIONS AND OPPORTUNITIES
Recent advancements in ENose technology include improved sensor technology and signal processing techniques, leading to more accurate and dependable ENose equipment for medical diagnosis. Integrating ENose technologies with other diagnostic modalities, such as imaging or genetic analysis, enables a more comprehensive and multi-modal approach to disease detection and monitoring. ENose technology is being applied in new areas like personalized medicine, disease phenotyping, therapeutic surveillance, and precision agriculture, demonstrating its adaptability and potential in various fields. These advancements and trends signify the ongoing evolution and maturation of ENose technology, paving the way for enhanced diagnostic capabilities, improved patient outcomes, and broader applications across different sectors. Beyond medical uses, ENose is used in the food sector for food safety and quality control, identifying spoilage, pollutants, and determining food freshness using odor profiles. These applications demonstrate the broad applicability and potential effect of ENose technology in a variety of sectors, highlighting its importance in developing diagnostics and improving patient outcomes[62-64]. Future trends in ENose technology include integration with artificial intelligence for improved diagnosis and personalized medicine. ENose has the potential to revolutionize healthcare by enabling early detection and targeted treatment strategies. Particularly, since ENose was developed for diagnosing various diseases and conditions, early cancer diagnosis is a primary purpose. It is conceivable that, based on the cancer site, different strategies might be adopted (e.g., breath for lung cancers and cancers heavily affecting metabolism like liver and kidney, bowel gas for GI tract tumors, and even blood for hematopoietic malignancies and metastasis in general). In fact, ENose has been shown to differentiate between cancer types based on VOC profiles. Furthermore, ENose might also help anticipate complications in cancer patients, such as infections, liver and kidney toxicity, or glucose level deregulation following steroid administration[65,66]. Additionally, ENose assesses exposure to environmental toxins and pollutants and detects toxins in air, water, or soil through VOC analysis. In the food and beverage industry, ENose ensures food safety and quality by detecting spoilage, contamination, and adulteration in food products and assessing the quality and authenticity of beverages based on aroma profiles. This may impact cancer epidemiology, favoring the recognition of risk factors and maybe preventing the consumption of contaminated food[67].
Role of ENose in remote cancer monitoring and telemedicine
ENoses play an important role in remote monitoring and telemedicine because they provide a
Collaborative efforts and interdisciplinary research in advancing ENose technology
ENose technology's future includes personalized medicine and point-of-care diagnostics, leveraging advanced sensors and AI for tailored healthcare. Its role extends to remote monitoring and telemedicine, enabling real-time data transmission and enhancing patient accessibility. Collaborative interdisciplinary research is crucial for advancing ENose capabilities, driving innovation, and integrating it into clinical practice for improved patient outcomes[73-75].
Clinical implementation
Several major recommendations can help to guide future ENose technology. To begin, ENose devices must continue to be validated and standardized in clinical applications. Robust clinical trials (phase 1 to 4 diagnostic accuracy studies) are required to prove the reliability, sensitivity, and specificity of ENose technology for diverse cancer types, and for different VOC sources, hence assuring its usefulness in
DISCUSSION AND CONCLUSION
The ENose technology has developed as a potential field in medical diagnostics and uses sensor arrays that simulate the human olfactory system to detect odors and volatile organic compounds (VOCs). ENose consists of sensor arrays composed of various sensors such as metal oxides and conductive polymers, which respond differently to various VOCs. Pattern recognition algorithms use these responses to create a unique “olfactory fingerprint” to identify and classify odors. The analysis presented in this paper explores the evolution, basic concepts, advantages, challenges, and future possibilities of ENose technology in the healthcare field, with a focus on cancer diagnosis. ENose technology has diverse medical applications. Beyond oncology, ENose has widespread applications in the management of diabetes, respiratory health, and infectious disease diagnosis, including respiratory diseases [asthma, chronic obstructive pulmonary disease (COPD), respiratory infections], infectious diseases (tuberculosis, pneumonia, sepsis), metabolic disorders (diabetes, kidney disease, liver disease), and neurological disorders (Alzheimer’s, Parkinson’s, epilepsy). For instance, this technology can differentiate between bacterial and viral infections with 87% accuracy, which is a crucial feature during pandemics like COVID-19. ENose is also increasingly used in food safety and environmental monitoring, where it can detect pollutants and food spoilage with high accuracy, indirectly contributing to disease prevention and public health promotion. Recent years have shown that cancer diagnosis can significantly benefit from ENose technology, both in early detection and post-treatment monitoring. For example, the World Health Organization (WHO) reported 19.3 million new cancer cases in 2020 and predicts this number will rise by around 50% by 2040, highlighting the urgent need for innovative technologies like
DECLARATIONS
Acknowledgments
The authors would like to express their sincere gratitude to the Robotics and Automation Research Laboratory (RARL) for their invaluable support throughout the course of this research.
Authors’ contributions
Conceptualization: Moshayedi AJ, Chen M, Piccaluga PP
Methodology, writing - original draft preparation: Moshayedi AJ, Khan AS, Piccaluga PP
Investigation, resources, writing - review and editing: Moshayedi AJ, Khan AS, Chen M, Piccaluga PP
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by Jiangxi University of Science and Technology, 341000, Ganzhou, P.R. China, underfunding number 2021205200100563.
Conflicts of interest
Piccaluga PP is an Editorial Board member of Journal of Cancer Metastasis and Treatment. Piccaluga PP was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, and decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Aly MT, Tasnim N, Najjaran H, Fardindoost S, Hoorfar M. Pattern recognition system for rapid detection of gases using microfluidic olfaction detector: a case study using methane and ethane. Sens Actuators B Chem. 2024;403:135201.
2. Shigaki S, Yamada M, Kurabayashi D, Hosoda K. Robust moth-inspired algorithm for odor source localization using multimodal information. Sensors. 2023;23:1475.
3. Li H, Yuan J, Yuan H. An active olfaction approach using deep reinforcement Learning for Indoor Attenuation Odor Source Localization. IEEE Sensors J. 2024;24:14561-72.
4. Jabeen M, Meng Q, Jing T, Hou H. Robot odor source localization in indoor environments based on gradient adaptive extremum seeking search. Build Environ. 2023;229:109983.
5. Qu X, Hu Y, Xu C, et al. Optical sensors of volatile organic compounds for non-invasive diagnosis of diseases. Chem Eng J. 2024;485:149804.
6. Javed R, Abbas T, Khan AH, Daud A, Bukhari A, Alharbey R. Deep learning for lungs cancer detection: a review. Artif Intell Rev. 2024;57:197.
7. Wijbenga N, Hoek RAS, Mathot BJ, et al. The potential of electronic nose technology in lung transplantation: a proof of principle. ERJ Open Res. 2022;8:00048-2022.
8. van Geffen WH, Lamote K, Costantini A, et al. The electronic nose: emerging biomarkers in lung cancer diagnostics. Breathe. 2019;15:e135-41.
9. Kokocińska-Kusiak A, Woszczyło M, Zybala M, Maciocha J, Barłowska K, Dzięcioł M. Canine olfaction: physiology, behavior, and possibilities for practical applications. Animals. 2021;11:2463.
10. van de Goor R, van Hooren M, Dingemans AM, Kremer B, Kross K. Training and validating a portable electronic nose for lung cancer screening. J Thorac Oncol. 2018;13:676-81.
11. Zakari S, Niels NK, Olagunju GV, et al. Emerging biomarkers for non-invasive diagnosis and treatment of cancer: a systematic review. Front Oncol. 2024;14:1405267.
12. Scheepers MHMC, Al-Difaie Z, Brandts L, Peeters A, van Grinsven B, Bouvy ND. Diagnostic performance of electronic noses in cancer diagnoses using exhaled breath: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2219372.
13. Lupan I, Silaghi C, Stroe C, et al. The importance of genetic screening on the syndromes of colorectal cancer and gastric cancer: a 2024 update. Biomedicines. 2024;12:2655.
14. Tyagi H, Daulton E, Bannaga AS, Arasaradnam RP, Covington JA. Non-invasive detection and staging of colorectal cancer using a portable electronic nose. Sensors. 2021;21:5440.
15. Borowik P, Adamowicz L, Tarakowski R, Siwek K, Grzywacz T. Odor detection using an E-Nose with a reduced sensor array. Sensors. 2020;20:3542.
16. Yan J, Zhang H, Ge X, Yang W, Peng X, Liu T. A novel bionic olfactory network combined with an electronic nose for identification of industrial exhaust. Microchem J. 2024;200:110287.
17. Lucchi G, Crépin M, Chambaron S, et al. Effects of psychological stress on the emission of volatile organic compounds from the skin. Sci Rep. 2024;14:7238.
18. Teodoro-Morrison T, Diamandis EP, Rifai N, et al. Animal olfactory detection of disease: promises and pitfalls. Clin Chem. 2014;60:1473-9.
19. Sakr R, Ghsoub C, Rbeiz C, et al. COVID-19 detection by dogs: from physiology to field application-a review article. Postgrad Med J. 2022;98:212-8.
20. Červený K, Janoušková K, Vaněčková K, et al. Olfactory evaluation in clinical medical practice. J Clin Med. 2022;11:6628.
21. Katotomichelakis M, Simopoulos E, Tripsianis G, et al. Improvement of olfactory function for quality of life recovery. Laryngoscope. 2013;123:E10-6.
22. Maniaci A, Lechien JR, Vaira LA, La Via L. Taste and smell disorders: a critical look at olfactory and gustatory dysfunction. Life. 2024;14:301.
23. Toussaint N, de Roon M, van Campen JP, Kremer S, Boesveldt S. Loss of olfactory function and nutritional status in vital older adults and geriatric patients. Chem Senses. 2015;40:197-203.
24. Oleszkiewicz A, Kunkel F, Larsson M, Hummel T. Consequences of undetected olfactory loss for human chemosensory communication and well-being. Philos Trans R Soc Lond B. 2020;375:20190265.
25. Oleszkiewicz A, Park D, Resler K, et al. Quality of life in patients with olfactory loss is better predicted by flavor identification than by orthonasal olfactory function. Chem Senses. 2019;44:371-7.
26. Fatuzzo I, Niccolini GF, Zoccali F, et al. Neurons, nose, and neurodegenerative diseases: olfactory function and cognitive impairment. Int J Mol Sci. 2023;24:2117.
27. Baldini C, Billeci L, Sansone F, Conte R, Domenici C, Tonacci A. Electronic nose as a novel method for diagnosing cancer: a systematic review. Biosensors. 2020;10:84.
28. Scheepers MHMC, Al-Difaie Z, Brandts L, Peeters A, van Grinsven B, Bouvy ND. Diagnostic performance of electronic noses in cancer diagnoses using exhaled breath: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2219372.
29. Li W, Jia Z, Xie D, Chen K, Cui J, Liu H. Recognizing lung cancer using a homemade e-nose: a comprehensive study. Comput Biol Med. 2020;120:103706.
30. Verschakelen JA, Bogaert J, De Wever W. Computed tomography in staging for lung cancer. Eur Respir J Suppl. 2002;19:40s-8s.
31. Rocco G, Pennazza G, Tan KS, et al. A real-world assessment of stage I lung cancer through electronic nose technology. J Thorac Oncol. 2024;19:1272-83.
32. Lee MR, Kao MH, Hsieh YC, et al. Cross-site validation of lung cancer diagnosis by electronic nose with deep learning: a multicenter prospective study. Respir Res. 2024;25:203.
33. Vadala R, Pattnaik B, Bangaru S, et al. A review on electronic nose for diagnosis and monitoring treatment response in lung cancer. J Breath Res. 2023;17:024002.
34. Machado RF, Laskowski D, Deffenderfer O, et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am J Respir Crit Care Med. 2005;171:1286-91.
35. Di Natale C, Macagnano A, Martinelli E, et al. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens Bioelectron. 2003;18:1209-18.
36. D’Amico A, Pennazza G, Santonico M, et al. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer. 2010;68:170-6.
37. Tran VH, Hiang Ping Chan, Thurston M, et al. Breath analysis of lung cancer patients using an electronic nose detection system. IEEE Sensors J. 2010;10:1514-8.
38. Peng G, Hakim M, Broza YY, et al. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer. 2010;103:542-51.
39. Bikov A, Hernadi M, Korosi BZ, et al. Expiratory flow rate, breath hold and anatomic dead space influence electronic nose ability to detect lung cancer. BMC Pulm Med. 2014;14:202.
40. Hubers AJ, Brinkman P, Boksem RJ, et al. Combined sputum hypermethylation and eNose analysis for lung cancer diagnosis. J Clin Pathol. 2014;67:707-11.
41. Tirzīte M, Bukovskis M, Strazda G, Jurka N, Taivans I. Detection of lung cancer in exhaled breath with an electronic nose using support vector machine analysis. J Breath Res. 2017;11:036009.
42. Tirzïte M, Bukovskis M, Strazda G, Jurka N, Taivans I. Detection of lung cancer with electronic nose and logistic regression analysis. J Breath Res. 2018;13:016006.
43. Huang CH, Zeng C, Wang YC, et al. A study of diagnostic accuracy using a chemical sensor array and a machine learning technique to detect lung cancer. Sensors. 2018;18:2845.
44. Berna A. Metal oxide sensors for electronic noses and their application to food analysis. Sensors. 10;10:3882-910.
45. Benet J, Seo M, Khine M, Gumà Padró J, Pardo Martnez A, Kurdahi F. Breast cancer detection by analyzing the volatile organic compound (VOC) signature in human urine. Sci Rep. 2022;12:14873.
46. Ramírez W, Pillajo V, Ramírez E, Manzano I, Meza D. Exploring components, sensors, and techniques for cancer detection via eNose technology: a systematic review. Sensors. 2024;24:7868.
47. Tyagi H, Daulton E, Bannaga AS, Arasaradnam RP, Covington JA. Development of electronic nose as a complementary screening tool for breath testing in colorectal cancer. Biosensors. 2025;15:82.
48. Zheng W, Pang K, Min Y, Wu D. Prospect and challenges of volatile organic compound breath testing in non-cancer gastrointestinal disorders. Biomedicines. 2024;12:1815.
49. Schuermans VNE, Li Z, Jongen ACHM, et al. Pilot study: detection of gastric cancer from exhaled air analyzed with an electronic nose in chinese patients. Surg Innov. 2018;25:429-34.
50. Capelli L, Bax C, Grizzi F, Taverna G. Optimization of training and measurement protocol for eNose analysis of urine headspace aimed at prostate cancer diagnosis. Sci Rep. 2021;11:20898.
51. Taverna G, Grizzi F, Bax C, et al. Prostate cancer risk stratification via eNose urine odor analysis: a preliminary report. Front Oncol. 2024;14:1339796.
52. Tagar MR, Soomro SP, Mastafa M, et al. Investigating the potential of non-invasive breath test analysis for early detection of oral cancer: a systematic review: non-invasive breath test for early oral cancer detection. PJHS. 2024;5:229-36.
53. Hooren MR, Leunis N, Brandsma DS, Dingemans AC, Kremer B, Kross KW. Differentiating head and neck carcinoma from lung carcinoma with an electronic nose: a proof of concept study. Eur Arch Otorhinolaryngol. 2016;273:3897-903.
54. Hakim M, Billan S, Tisch U, et al. Diagnosis of head-and-neck cancer from exhaled breath. Br J Cancer. 2011;104:1649-55.
55. Gruber M, Tisch U, Jeries R, et al. Analysis of exhaled breath for diagnosing head and neck squamous cell carcinoma: a feasibility study. Br J Cancer. 2014;111:790-8.
56. Goertzen A, Kidane B, Ahmed N, Aliani M. Potential urinary volatile organic compounds as screening markers in cancer - a review. Front Oncol. 2024;14:1448760.
57. Tyagi H, Daulton E, Bannaga AS, Arasaradnam RP, Covington JA. Electronic nose for bladder cancer detection†. Chem Proc. 2021;5:22.
58. Anzivino R, Sciancalepore PI, Dragonieri S, et al. The role of a polymer-based E-Nose in the detection of head and neck cancer from exhaled breath. Sensors. 2022;22:6485.
59. Blanchet L, Smolinska A, Baranska A, et al. Factors that influence the volatile organic compound content in human breath. J Breath Res. 2017;11:016013.
60. Berkhout DJC, Benninga MA, de Boer NKH, de Meij TG. Revival of an ancient greek art: scent detection as diagnostic tool for tuberculosis. Pediatr Res. 2018;84:4-5.
61. Ahmad B, Masoud MS, Ashfaq UA, Ansari M-ur-R, Nahid N, Qasim M. Smart E-noses for medical and clinical applications. In: Advanced Sensors for Smart Healthcare. Elsevier; 2025. pp. 231-42.
62. Selvaraj R, Vasa NJ, Nagendra SMS, Mizaikoff B. Advances in mid-infrared spectroscopy-based sensing techniques for exhaled breath diagnostics. Molecules. 2020;25:2227.
63. Pleil JD, Stiegel MA, Sobus JR, Liu Q, Madden MC. Observing the human exposome as reflected in breath biomarkers: heat map data interpretation for environmental and intelligence research. J Breath Res. 2011;5:037104.
64. Sundar KM, Stark A, Morris MJ. Laryngeal dysfunction manifesting as chronic refractory cough and dyspnea: laryngeal physiology in respiratory health and disease. Chest. 2024;166:171-86.
65. Moshayedi AJ, Khan AS, Shuxin Y, et al. E-Nose design and structures from statistical analysis to application in robotic: a compressive review. EAI Endorsed Trans AI Robotics. 2023;2:e1.
66. Albastaki Y. Clustering algorithms as a tool for odour classifications in enose developments. In: Musleh Al-sartawi AM, Razzaque A, Kamal MM, editors. Artificial intelligence systems and the internet of things in the digital era. Cham: Springer International Publishing; 2021. pp. 46-56.
67. Teixeira GG, Peres AM, Estevinho L, et al. Enose lab made with vacuum sampling: quantitative applications. Chemosensors. 2022;10:261.
69. Liu X, Cheng S, Liu H, Hu S, Zhang D, Ning H. A survey on gas sensing technology. Sensors. 2012;12:9635-65.
70. Oates MJ, Gonzalez-teruel JD, Ruiz-abellon MC, Guillamon-frutos A, Ramos JA, Torres-sanchez R. Using a low-cost components e-Nose for basic detection of different foodstuffs. IEEE Sensors J. 2022;22:13872-81.
71. Bosch S, de Menezes RX, Pees S, et al. Electronic nose sensor drift affects diagnostic reliability and accuracy of disease-specific algorithms. Sensors. 2022;22:9246.
72. Buma AIG, Muller M, de Vries R, et al. eNose analysis for early immunotherapy response monitoring in non-small cell lung cancer. Lung Cancer. 2021;160:36-43.
73. Binson VA, Subramoniam M, Mathew L. Prediction of lung cancer with a sensor array based e-nose system using machine learning methods. Microsyst Technol. 2024;30:1421-34.
74. Vries R, Sterk PJ. eNose breathprints as composite biomarker for real-time phenotyping of complex respiratory diseases. J Allergy Clin Immunol. 2020;146:995-6.
75. Wilson AD. Noninvasive early disease diagnosis by electronic-nose and related VOC-detection devices. Biosensors. 2020;10:73.
76. Wilson AD. Recent applications of electronic-nose technologies for the noninvasive early diagnosis of gastrointestinal diseases†. Proceedings. 2018;2:147.
77. Behera B, Joshi R, Anil Vishnu GK, Bhalerao S, Pandya HJ. Electronic nose: a non-invasive technology for breath analysis of diabetes and lung cancer patients. J Breath Res. 2019;13:024001.
78. Wasilewski T, Migoń D, Gębicki J, Kamysz W. Critical review of electronic nose and tongue instruments prospects in pharmaceutical analysis. Anal Chim Acta. 2019;1077:14-29.
79. Alfieri G, Modesti M, Riggi R, Bellincontro A. Recent advances and future perspectives in the E-nose technologies addressed to the wine industry. Sensors. 2024;24:2293.
80. Wilson AD, Baietto M. Applications and advances in electronic-nose technologies. Sensors. 2009;9:5099-148.
81. Ahmad B, Ashfaq UA, Mahmood-ur-rahman, et al. E-nose-based technology for healthcare. In: Nanotechnology-Based E-noses. Elsevier; 2023. pp. 241-56.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].