The application value of preoperative F-FDG PET/CT in preoperative localized clear cell renal cell carcinoma
Abstract
Aim: In cancer tissues, glycolysis metabolism is often heightened, making 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) useful for assessing glucose metabolism. However, the kidneys' high glucose metabolism makes it difficult to distinguish between normal renal tissue and renal cancer. This study aims to evaluate the maximum standardized uptake value (SUVmax) in renal cancer using PET/CT and determine its relationship to prognosis.
Methods: We also aim to examine the correlation between SUVmax and clinical parameters and its potential link to prognosis. We enrolled 105 patients who underwent FDG-PET/CT between March 2012 and October 2017. These patients, diagnosed with localized renal cell carcinoma (RCC), underwent surgery and were pathologically confirmed to have clear cell RCC (ccRCC). We investigated the impact of SUVmax and other parameters on recurrence.
Results: SUVmax and C-reactive protein (CRP) were associated with tumor progression, alongside stage, nuclear grade, microvascular invasion [v(+)], and tumor-infiltrating lymphocytes (TILs). Multivariate analysis with recurrence-free survival (RFS) as the endpoint indicated significant results (SUVmax ≥ 3.7: relative risk 10.21, P < 0.01; CRP ≥ 0.11 mg/dL: relative risk 7.73, P < 0.01, n = 89). In survival curve analysis with RFS, high SUVmax or elevated CRP predicted poor prognosis, with further worsening when v(+) was added.
Conclusion: SUVmax is a strong prognostic factor for poor outcomes. CRP is also a prognostic factor, though it should be interpreted cautiously, as CRP reflects overall systemic conditions and may not exclusively represent renal cancer activity. Further research into pre-treatment RCC prognosis prediction is anticipated.
Keywords
INTRODUCTION
In general, cancer is a disease that significantly affects life prognosis. While advancements in surgical and pharmacological therapies are naturally expected as part of treatment strategies, establishing prognostic biomarkers to guide treatment decisions is equally important. Recently, Sahin et al. examined the relationship between cancer patient survival rates and the Royal Marsden Hospital (RMH) score, a prognostic tool based on clinical information applicable to patients with various types of cancer[1]. They found that even in subgroup analyses of different cancer types, cases with high RMH scores consistently showed reduced overall survival rates. On the other hand, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) is a tool for the diagnosis and treatment evaluation of various cancers and active inflammatory diseases[2-4]. Specifically, it provides insights into effectiveness and disease details through imaging of tissue metabolism. Recently, its applications have been expanding. As of 2020, renal cell carcinoma (RCC) ranks as the 14th most common cancer globally, with approximately 300,000 new cases annually, and it causes 100,000 deaths each year[5,6]. Notably, 75% of RCC cases are of the clear cell subtype. However, the application of FDG PET/CT in kidney cancer remains limited. This is primarily because the kidneys are metabolically active organs, making it challenging to distinguish renal parenchyma and urinary tract from renal cancer lesions on PET/CT scans[7]. Considering that kidney cancer responds to novel treatments such as molecular targeted therapies and immunotherapies, and that there are also local treatment options like Radiofrequency Ablation (RFA) and Cryoablation (CA), it may be worth reevaluating the utility of PET/CT in kidney cancer as a marker to guide treatment strategies, similar to its role in other types of cancer. Recent evidence has emerged to overcome the challenges of using PET/CT in the kidney, although it remains limited. At our institution, extensive research has been conducted on the metabolic characteristics and FDG uptake in both normal and cancerous renal tissues[8,9]. Moreover, a growing body of evidence suggests a link between cancer, immunity, and inflammation[10,11]. In RCC, parameters such as C-reactive protein (CRP) (for systemic evaluation), maximum standardized uptake value (SUVmax) (for local evaluation), and tumor-infiltrating lymphocytes (TILs) are gaining attention for their potential associations with cancer prognosis[12-14]. Therefore, we believe that it is meaningful to comprehensively examine these parameters in RCC. The primary objective of this study is to determine whether FDG uptake is associated with the prognosis of patients with localized clear cell RCC (ccRCC) who underwent preoperative PET imaging. As secondary objectives, the study will investigate whether FDG uptake correlates with existing parameters (such as pathological findings) and inflammation-related factors [including CRP, neutrophil-to-lymphocyte ratio (NLR), and TILs]. If correlations are found, the relationship between these factors and prognosis will also be examined. Based on the above research plan, which hypothesizes a correlation between SUVmax and CRP, the results will be incorporated to discuss the potential preoperative applications of these factors and their molecular biological relationship with each other.
METHODS
Patients
From March 2012 to October 2017, a total of 105 patients were enrolled in the study who underwent imaging, including FDG-PET/CT, and were diagnosed with RCC followed by surgical treatment. All cases were pathologically confirmed as ccRCC [Table 1]. Notably, cases with a history of chronic inflammatory diseases, regular steroid use, other active malignancies or those who received adjuvant therapy preoperatively or postoperatively were excluded from the study to avoid potential confounding effects on the inflammatory markers being analyzed. This exclusion criterion ensures that the findings regarding the correlation between FDG uptake and inflammatory parameters, as well as their potential impact on prognosis, are not biased by underlying inflammatory conditions. This study complied with the Declaration of Helsinki and was approved by the ethical committee of Dokkyo Medical University Hospital, and all enrolled cases provided written informed consent.
Patient and tumor characteristics
| Characteristics | |
| Patients | 105 |
| Age (years) | 67(30-88) |
| Gender (male/female) | 74/31 |
| Follow-up duration (months) | 78(1-139) |
| Body mass index | 24.2(17.9-36.6) |
| Tumors | |
| pStage (I/II/III) | 82/3/20 |
| Fuhrman grade (G1/G2/G3/G4) | 25/70/7/3 |
| Microvascular invasion (-/+) | 67/38 |
| TILs score (1/2/3) | 77/24/4 |
| Nephrectomy (radical/partial) | 77/28 |
| Recurrence (local/metastasis) | 21(3/18) |
Methods
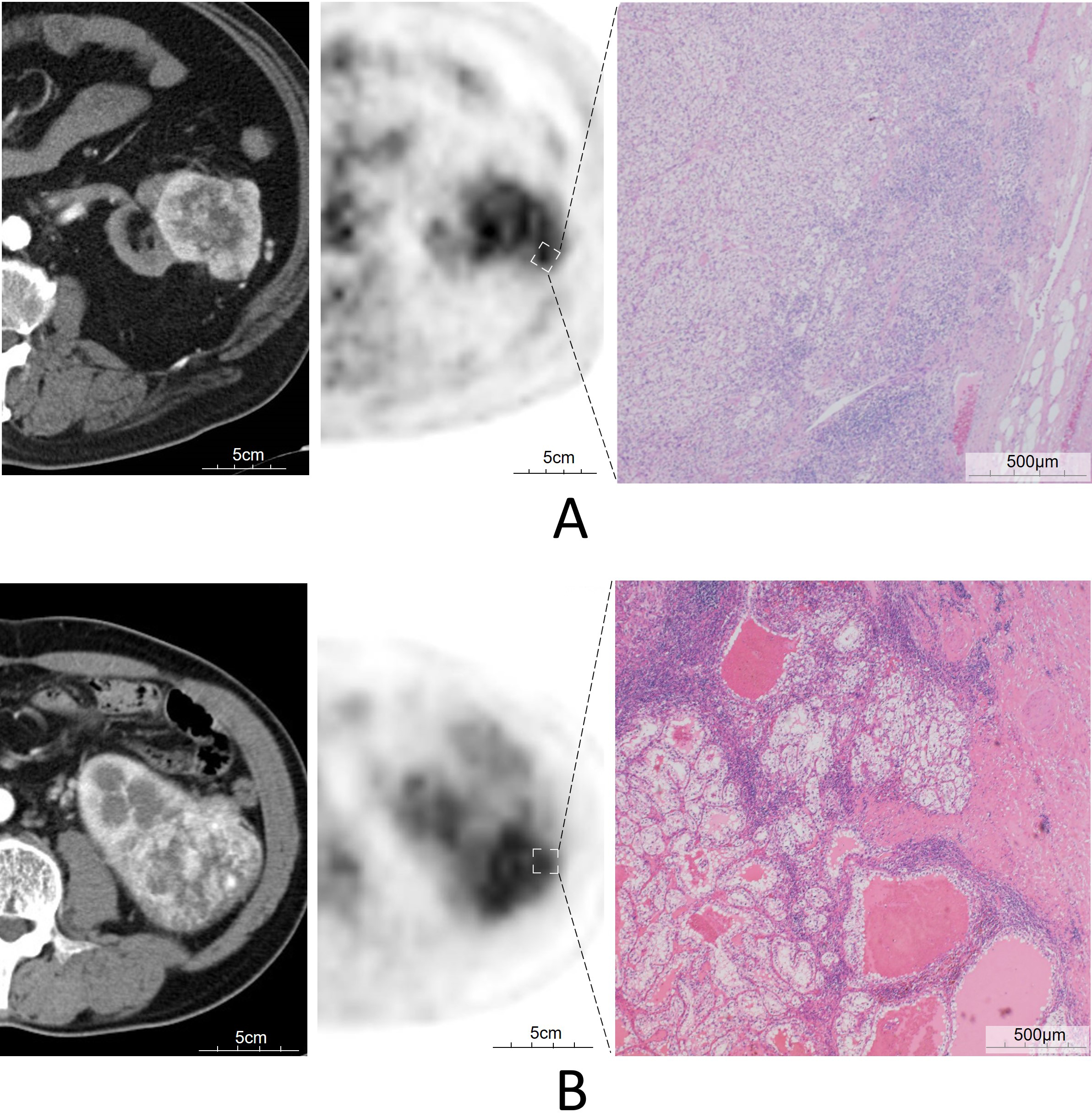
Pathological findings: Tumor histological classification and staging were conducted using the Fuhrman grading system and the tumor-lymph node-distant metastasis (TNM) classification[15,16]. These established systems provide a standardized approach for evaluating the malignancy and progression of RCC, facilitating consistent and comparable analysis across cases. TILs Scoring Methodology: The TILs score was measured using hematoxylin and eosin-stained specimens from surgically resected tissues. With reference to the study by Salgado et al.[17], we scored stromal TILs within cancer lesions by measuring the total stromal area within the lesion as the denominator and the TILs within the stroma as the numerator, excluding necrotic areas and regions with granulocyte localization. Based on the level of TILs observed, specimens were categorized into scoring 1 (< 10%; n = 77), scoring 2 (≥ 10% to < 50%; n =24), and scoring 3 (≥ 50%; n = 4) [Table 1 and Figure 1]. The pathological assessment was validated by two board-certified pathologists to ensure accuracy and reliability.
Figure 1. FDG PET/CT images of two patients with ccRCC, Axial CT, arterial phase (left), fused PET/CT (middle), microscopic findings with TILs (right), (A) images of a 59-year-old-man, pT1b, G2; SUVmax was 6.1, TILs scoring 2; and (B) images of a 57-year-old man, pT3a, G2; SUVmax was 7.1, TILs scoring 3. FDG PET/CT: 18F-fluorodeoxyglucose positron emission tomography/computed tomography; ccRCC: clear cell RCC; RCC: Renal cell carcinoma; TILs: Tumor-infiltrating lymphocytes; SUVmax: Maximum standardized uptake value.
NLR and CRP: Preoperative blood test data, including CRP and complete blood count, were obtained from the patients' medical records within four weeks before surgery. The NLR was calculated by dividing the number of neutrophils by the number of lymphocytes, as previously described. This parameter serves as a marker of systemic inflammation, which has been linked to cancer prognosis in various studies[18,19].
SUVmax calculation: Patients generally received an intravenous injection of FDG (222-333MBq) after fasting for at least 6 h, with 4.5 MBq/kg. Whole-body imaging using a combined FDG-PET/CT scanner, Biograph Sensation 16 (Siemens, Erlangen, Germany), and evaluation of the images were performed according to our previously reported methodology[9]. CT scanner covered a region ranging from the head to the mid-thigh. After 1 h later, CT scanning was conducted and whole-body PET scanning was performed with acquisition for 3 min per bed position using the three-dimensional acquisition mode. The SUV was determined according to the standard formula, with activity in the volume-of-interest (VOI) being calculated as Bq/ml divided by the injected dose in Bq/kg. In each patient, the average SUV was calculated from all images obtained about 1 h after tracer injection. SUVmax was defined as the maximum activity within the VOI using Syngo.via (Siemens Healthcare, Forchheim, Germany). We measured SUVmax of the lesions in reference to four-phase dynamic enhanced CTs (non-contrast, corticomedullary, parenchymal, and excretory phases), avoiding renal parenchyma and urinary tract [Figure 1]. We also decided on the interval between FDG-PET and enhanced CTs within three weeks. The calculated values were reviewed by two certified PET/CT specialists to ensure consistency and accuracy. Notably, there were 16 cases with non-cancerous lesions showing higher FDG uptake than the renal cancer lesions [Table 2].
The cases exhibited higher FDG uptake than the renal cancer lesions (n = 16)
| Cases | FDG accumulation lesion: value of SUVmax | CRP (mg/dL) | |||
| RCC | Another organ | Suspicious disease | |||
| 1 | 4.3 | 4.8 | Spine (S1) | Degeneration | 0.35 |
| 2 | 4.6 | 17.9 | Ascending colon | Non-specific accumulation | 0.17 |
| 3 | 3.5 | 3.6 | Prostate | Inflammation | 0.24 |
| 4 | 3.9 | 4.3 | Spine (L3/4) | Degeneration | 0.35 |
| 5 | 2.4 | 3.7 | Maxillary sinus | Inflammation | 0.32 |
| 6 | 3.0 | 4.7 | Stomach | Non-specific accumulation | 0.02 |
| 7 | 2.7 | 15.4 | Parotid gland | Warthin tumor | 0.23 |
| 8 | 3.8 | 7.4 | Cervical lymph node | Non-specific accumulation | 0.16 |
| 9 | 1.9 | 4.1 | Spine (L5/S1) | Degeneration | 0.06 |
| 10 | 2.6 | 4.8 | Prostate | Inflammation | 1.04 |
| 11 | 3.4 | 9.3 | Thyroid gland | Inflammation | 0.04 |
| 12 | 3.2 | 5.7 | Mesenteric lymph node | Non-specific accumulation | 0.33 |
| 13 | 2.3 | 10.1 | Thyroid gland | Inflammation | 0.06 |
| 14 | 5.7 | 8.9 | Anus | Hemorrhoid | 0.04 |
| 15 | 1.7 | 10.9 | Thyroid gland | Inflammation | 0.46 |
| 16 | 2.4 | 6.2 | Alveolar bone | Inflammation | 0.11 |
| Median | 3.1 | 6.0 | 0.20 | ||
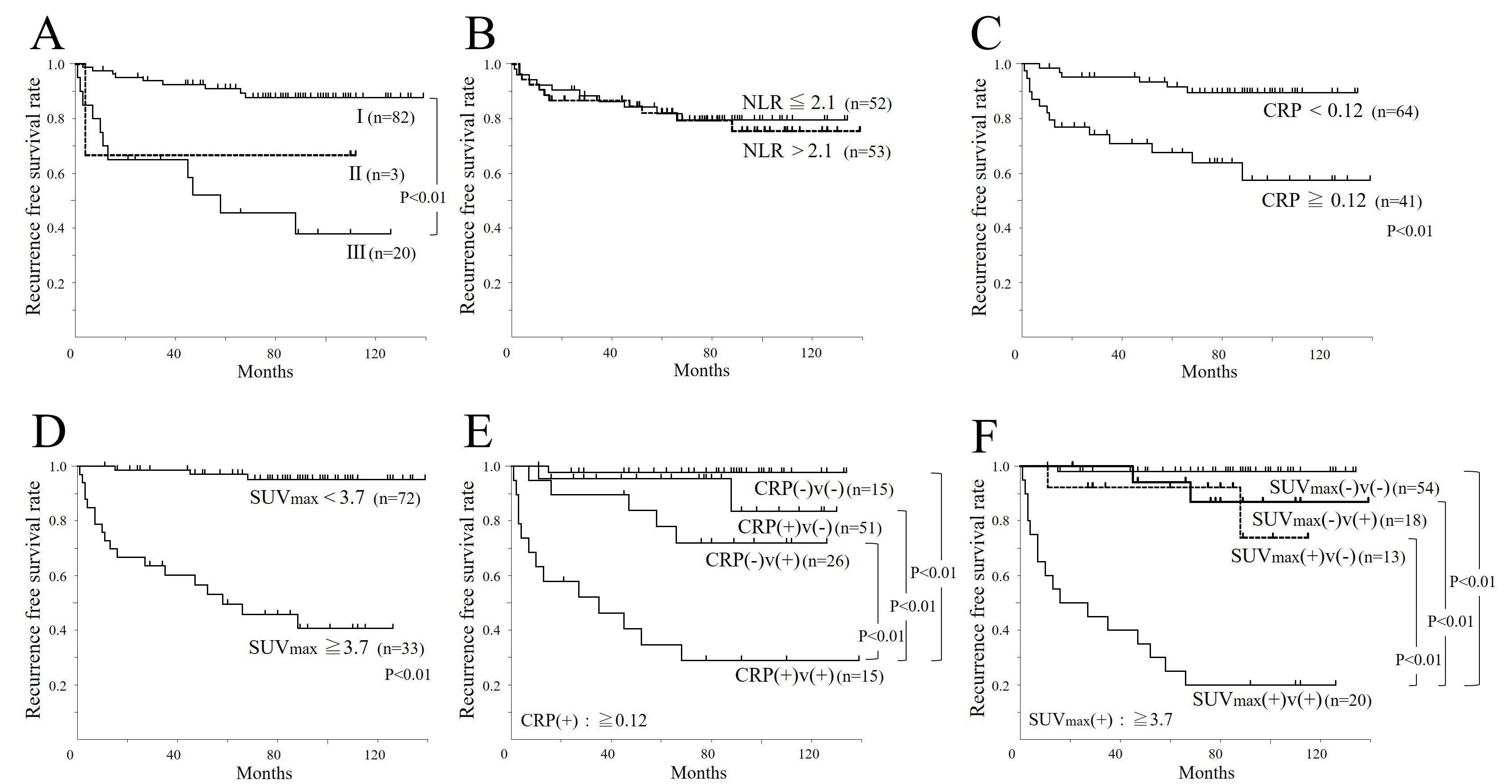
Statistical analysis: Establishing Cutoff Values: Median values were used for age and BMI, while cutoff values for NLR, CRP, and SUVmax were determined using receiver operating characteristic (ROC) curves. Kaplan-Meier curves and the log-rank test were employed to assess relapse-free survival across groups stratified by the identified cutoff values. These cutoff points were determined using a ROC curve, after which the patients were categorized into two groups. NLR, CRP, and SUVmax were categorized into two groups using the binary variable as a check of clinical validity by ROC analysis and a sensitivity-specificity curve because the results of Cox proportional-hazards regression analyses using continuous variable and binary variable showed no great difference. When NLR of 2.1, CRP of 0.12 (mg/dL), and SUVmax of 3.7 were used as cutoff points, the sensitivity, specificity, and area under the curve (AUC) of the ROC curves were 47.9%, 50.0%, and 0.543; 72.2%, 72.2%, and 0.765; and 81.7%, 83.3%, and 0.853, respectively [Figure 2]. Kaplan-Meier Analysis and Harrell’s C-index for recurrence-free survival (RFS): To account for the cases where PET/CT was not performed, the Kaplan-Meier method and Harrell’s C-index were used to analyze RFS based on various parameters in the initial cohort of 105 cases. This comprehensive analysis allows for a better understanding of the prognostic value of PET/CT and other markers in ccRCC. The relationship between continuous variables such as NLR, CRP, SUVmax, and the clinical stage and pathological findings was then analyzed [Figure 3]. Additionally, as shown in [Table 2], 16 cases exhibited higher FDG uptake than the renal cancer lesions. These cases were excluded from further analysis, and cutoff values were
Figure 2. Recurrence-free survival curves (n = 105). (A) pStage; (B) NLR: lower group ≦ 2.1, higher group > 2.1; (C) CRP: lower group < 0.12(mg/dL), higher group ≥ 0.12(mg/dL); (D) SUVmax: lower group < 3.7, higher group ≥ 3.7; (E) CRP + v-factor: bottom group as CRP(+) + v(+), lower group as CRP(-) + v(+), upper group as CRP(+) + v(-), top group as CRP(-) + v(-) [CRP cut-off degree is same as C]; (F) SUVmax + v-factor: bottom group as SUVmax(+) + v(+), lower group as SUVmax(+) + v(-), upper group as SUVmax(-) + v(+), top group as SUVmax(-) + v(-) [SUVmax cuf-off degree is same as D]. NLR: neutrophil-to-lymphocyte ratio; CRP: C-reactive protein; v: Microvascular invasion; SUVmax: Maximum standardized uptake value.
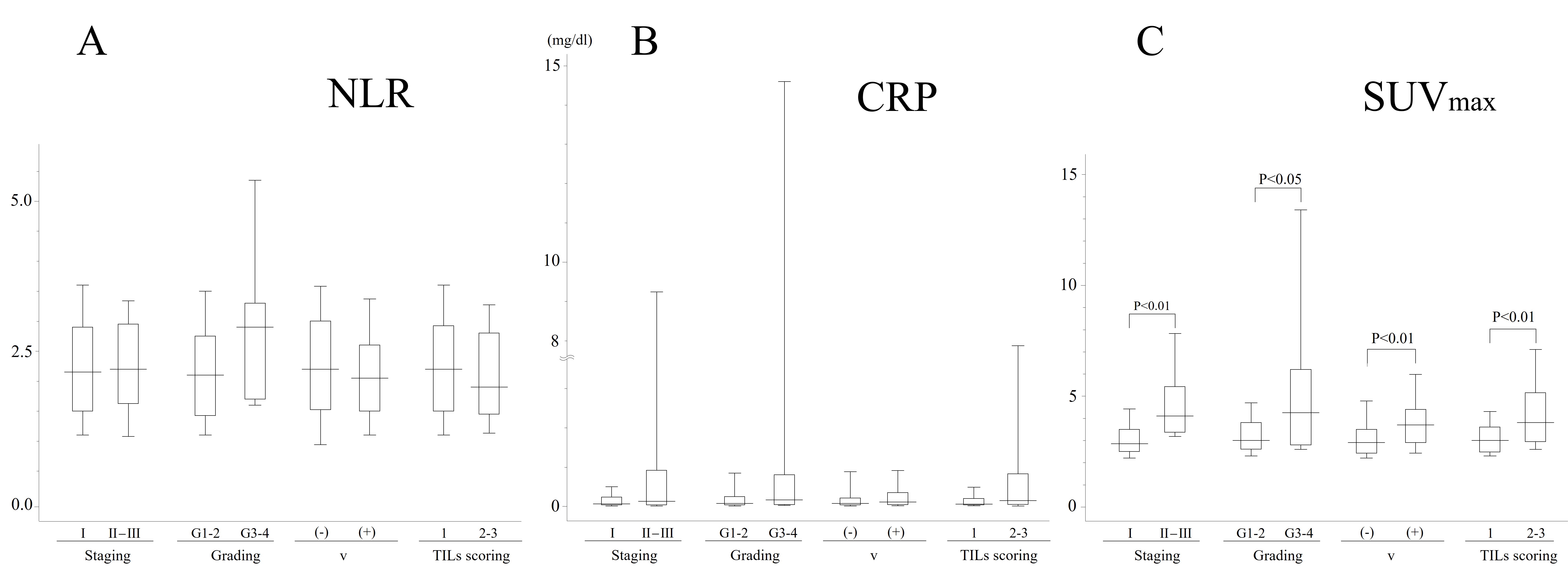
Figure 3. Correlation of NLR (A); CRP (B); and SUVmax values (C) with staging, grading, and TILs scoring (n = 105). Statistical significance was evaluated by Student’s t-test (A). Statistical significance was evaluated by Mann-Whitney U-test (B) and (C). Boxes, 25%-75% quartiles; horizontal lines, group medians; peak and minimal lines, 2.5%-97.5%. NLR: neutrophil-to-lymphocyte ratio; CRP:
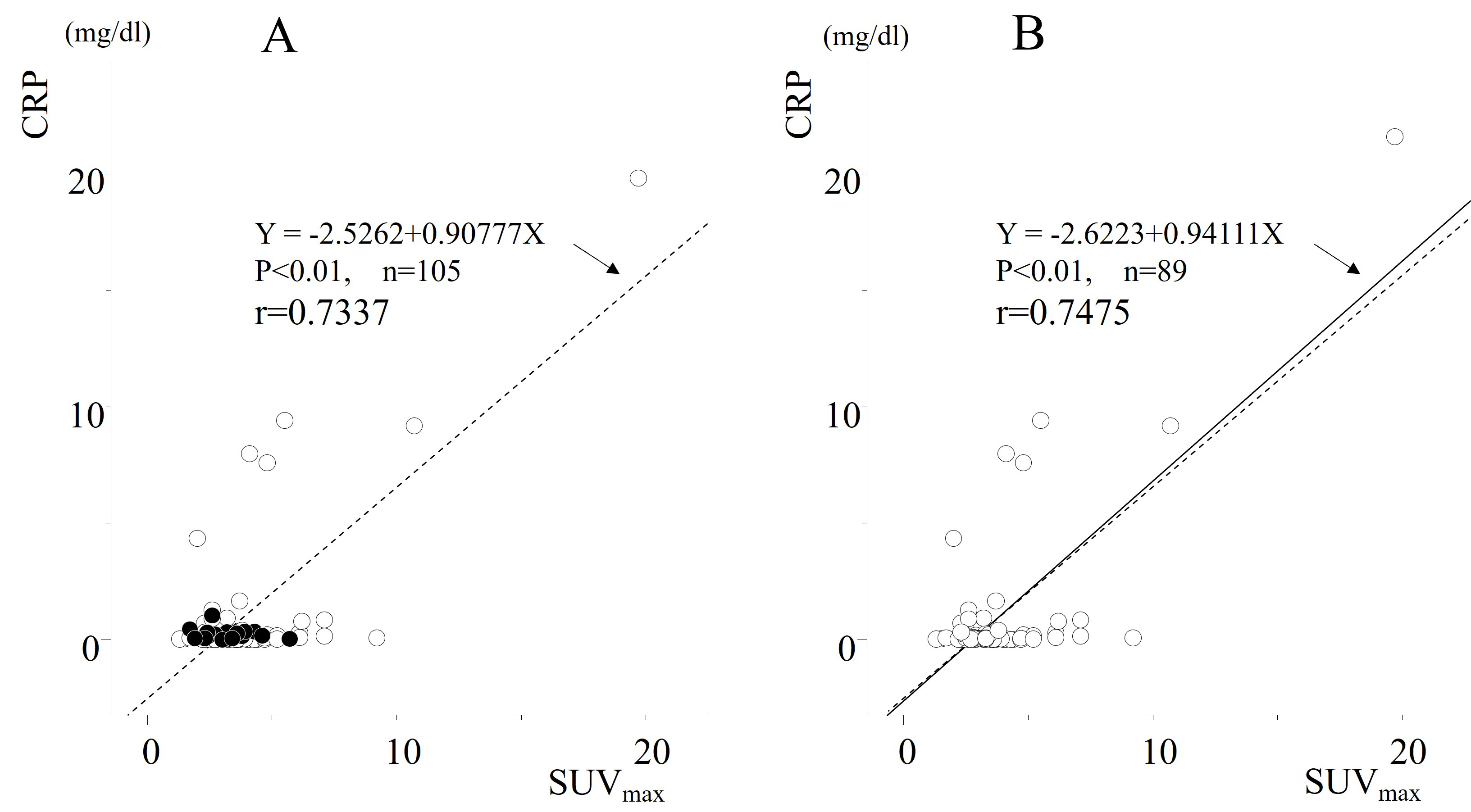
Figure 4. Spearman’s rank correlation of SUVmax values with CRP (mg/dL). (A) All cases (n = 105). Sixteen non-renal cancer lesions with higher FDG uptake than renal cancer lesions were identified, as indicated by black circles; (B) All cases (n = 89), excluding 16 cases where FDG uptake was higher in non-cancerous renal tissues than in RCC lesions. SUVmax: Maximum standardized uptake value; CRP: C-reactive protein; FDG: 18F-fluorodeoxyglucose.
Univariate and multivariate analyses for cancer-related recurrence-free survival of ccRCC (n = 89)
| Variable | Case number | Univariate analysis | Multivariate analysis1 | Multivariate analysis2 | ||||||
| P-Value | Relative risk (95%CI) | P-Value | Relative risk (95%CI) | P-Value | Relative risk (95%CI) | |||||
| Age < 67 (years) | 42 | 0.24 | 1.76 | (0.68-4.55) | 0.09 | 2.77 | (0.86-9.00) | 0.61 | 1.38 | (0.39-4.90) |
| Sex: male | 62 | 0.17 | 2.38 | (0.69-8.22) | 0.12 | 4.00 | (0.69-23.18) | 0.23 | 2.48 | (0.56-11.08) |
| BMI ≥ 24.2 | 45 | 0.20 | 1.90 | (0.71-5.06) | 0.49 | 0.60 | (0.14-2.58) | 1.00 | 1.00 | (0.25-4.04) |
| NLR < 2.1 | 43 | 0.98 | 1.01 | (0.40-2.55) | 0.50 | 1.44 | (0.50-4.09) | 0.34 | 1.77 | (0.54-5.82) |
| TILs score: 2,3 | 27 | < 0.05 | 2.75 | (1.09-6.92) | 0.32 | 1.71 | (0.60-4.89) | 0.40 | 1.64 | (0.51-5.21) |
| Grade: high grade(3,4) | 9 | < 0.05 | 3.19 | (1.05-9.73) | 0.97 | 0.97 | (0.23-4.22) | 0.79 | 0.80 | (0.15-4.23) |
| Surgery: nephrectomy | 65 | < 0.05 | 7.60 | (1.01-57.16) | 0.67 | 1.65 | (0.17-16.03) | 0.78 | 1.41 | (0.13-15.24) |
| Stage: II,III | 20 | < 0.01 | 5.81 | (2.29-14.76) | < 0.05 | 4.59 | (1.29-16.33) | 0.38 | 1.73 | (0.50-5.96) |
| Micrometastasis | 32 | < 0.01 | 10.81 | (3.13-37.37) | < 0.01 | 7.28 | (1.62-32.71) | < 0.05 | 5.62 | (1.37-23.07) |
| CRP ≥ 0.11 (mg/dL) | 33 | < 0.01 | 5.41 | (1.92-15.19) | < 0.01 | 7.73 | (2.41-24.79) | |||
| SUV ≥ 3.7 | 28 | < 0.01 | 15.55 | (4.48-53.93) | < 0.01 | 10.21 | (2.48-42.00) | |||
FDG imaging visualizes the glycolytic status by representing the intensity of FDG uptake in a visual format, in conjunction with fused images from CT. Since the kidneys excrete FDG via urine, distinguishing FDG uptake caused by actual lesions can be challenging when cancerous lesions are located near the renal medulla. This difficulty arises due to the spatial resolution limitations inherent to FDG-PET. In such cases, performing contrast-enhanced CT imaging can help differentiate FDG uptake originating from true lesions from other sources of uptake.
Among the 105 enrolled cases, it was found that in 16 cases, the SUVmax of non-cancerous lesions was higher than that of primary cancer lesions. Since SUVmax also reflects the degree of inflammation, it is challenging to conclude that the CRP value accurately reflects the primary lesions in such cases. Therefore, these 16 cases were excluded, and multivariate analysis was conducted on the remaining 89 cases. Furthermore, in Figure 4, CRP and SUVmax are likely confounding factors; hence, an analysis incorporating them was not performed, and two separate analyses were conducted instead.
RESULTS
The RFS curve represents the natural progression according to the disease stage [Figure 2A]. This figure highlights how different stages of RCC influence patient outcomes, providing a baseline for understanding the impact of other factors such as metabolic and inflammatory markers. In the analysis of the association between continuous variables (NLR, CRP, and SUVmax) and disease stage or pathological findings, no significant differences were observed for NLR and CRP, whereas SUVmax showed significant differences across all parameters [Figure 3A-C]. This suggests that SUVmax may be a more sensitive indicator of disease severity compared to NLR and CRP in RCC patients. When analyzing the association between various parameters (NLR, CRP, and SUVmax) and RFS using Kaplan-Meier curves, no significant difference was found for NLR, consistent with the results of bivariate analysis. However, high levels of CRP and SUVmax were associated with poor prognosis [Figure 2B-D]. The Harrell’s C-index results and 95% confidence intervals for each variable are as follows: (A) pStage: 0.694 (0.596-0.792); (B) NLR (no variables were retained); (C) CRP: 0.695 (0.605-0.786); (D) SUVmax: 0.811 (0.744-0.878); (E) CRP with the v factor: 0.831 (0.751-0.910); (F) SUVmax with the v factor: 0.836 (0.761-0.911) [Figure 2A-F]. This finding reinforces the potential prognostic value of CRP and SUVmax in predicting RCC outcomes.
Subsequently, referring to [Table 2], we conducted a correlation analysis between CRP and SUVmax (n = 89), excluding 16 cases where FDG uptake was higher in non-cancerous renal tissues than in RCC lesions. The correlation between CRP and SUVmax became more evident [Figure 4]. Based on these results, univariate and multivariate analyses were performed to assess the association between various parameters and RFS in the remaining 89 cases. In multivariate analysis, considering that SUVmax and CRP are confounding factors, two patterns were performed [Table 3]. TILs did not emerge as an independent prognostic factor. Given these findings, SUVmax, along with CRP and v-factor, were identified as independent prognostic factors in that order[Table 3]. Although CRP and v-factor were calculated postoperatively, it was observed that the combination of high CRP and v(+) or high SUVmax and v(+) resulted in extremely low non-recurrence rates beyond 80 months, approximately 30% and 20%, respectively [Figure 2E and F]. This highlights the importance of considering these factors in postoperative monitoring and management strategies.
DISCUSSION
The postoperative outcomes for localized ccRCC in our study were comparable to those reported by Zganjar et al., with a 5-year recurrence-free survival rate of 82.0% (95%CI: 78.1-84.1) in our cohort (n = 105) versus 76% (95%CI: 74-77) in their larger cohort (n = 1,967)[20]. This suggests that our findings related to surgery are highly reliable. While elevated NLR has been identified as an independent prognostic factor in various cancers[19,21,22], our study, consistent with other research on non-metastatic clear cell RCC, did not find significant results in this regard[18]. However, CRP, a marker of systemic inflammation, was confirmed to be a prognostic factor, in line with previous studies[13]. FDG uptake, representing metabolic activity in both inflammation and tumor lesions[23], is often correlated with prognosis in malignancies through the SUVmax[2,19]. In renal cell carcinoma, CRP has been recognized as a prognostic factor, but SUVmax has not gained the same recognition. This discrepancy may be due to the ease of measuring CRP and its clear correlation with prognosis[13], whereas SUVmax often presents challenges due to the typically low FDG uptake in many cancerous lesions and higher uptake in surrounding normal kidney tissue, leading to low contrast and less prognostic relevance[7,9]. However, serum CRP levels provide information only about systemic inflammation, making it challenging to discuss the relationship between cancerous lesions and CRP when both cancerous lesions and non-cancerous inflammatory diseases coexist. In this regard,
Strength and limitations
At the beginning of the analysis, it was confirmed that both SUVmax and CRP were individually strong prognostic factors and exhibited a strong correlation with each other. Therefore, multivariate analysis was conducted, considering the possibility of these two factors being confounding variables. As a result, SUVmax was identified as a stronger prognostic factor than CRP.
In this study, the inclusion criteria required that cases involve localized cancer at both preoperative and postoperative diagnosis, a histological diagnosis of clear cell carcinoma, preoperative FDG PET/CT, and no renal cancer treatment (e.g., medication or additional surgery) prior to the detection of recurrence. No intentional manipulation was performed during case accumulation. Cases were consecutively assigned retrospectively, totaling 105 cases, suggesting that validation in a larger cohort is desirable. Regarding the implementation of FDG PET/CT, our institution has made every effort to perform FDG PET/CT on patients referred for consideration of renal cancer surgery, regardless of the study period. Therefore, similar results are expected in further validation studies, regardless of whether PET scans were performed. It is also known that the prognosis of renal cancer varies depending on its histological classification. While this study focused on clear cell carcinoma, the most common type, this point should be carefully considered in validation studies and comparisons with other institutions. Additionally, this study did not address the correlation between SUVmax and inflammatory mediators other than CRP. Further investigation on this point is also warranted.
Conclusion
SUVmax is a powerful prognostic factor, and performing PET/CT at the initial diagnosis of clear cell renal cell carcinoma enables prognosis prediction even before surgery. These results can inform decisions regarding perioperative adjuvant therapy and postoperative follow-up strategies. While CRP is also a useful prognostic indicator as an alternative to SUVmax, it is crucial to consider that CRP reflects systemic inflammation and may not exclusively indicate renal cell carcinoma activity.
DECLARATIONS
Acknowledgments
The Authors thank Masaya Seki for his skillful technical assistance.
Authors’ contributions
Concept development and manuscript preparation: Betsunoh H, Kamai T
Data generation: Betsunoh H, Takada-Owada A, Sakamoto S, Nakagami Y, Ishida K
Experimental design: Betsunoh H, Yuki H, Nukui A, Hayashi K
Data analysis: Betsunoh H
All Authors cooperated in the internal review of the manuscript prior to submission.
Availability of data and materials
The data supporting the findings of this study can be obtained from the corresponding author upon reasonable request.
Financial support and sponsorship
This work was supported by the Research Support Award 2024 of Dokkyo International Medical Education and Research Foundation (R5-08).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics review board of Dokkyo Medical University Hospital (R-31-10J, 16 January 2020). Written informed consent was obtained from all study patients or their family members.
Consent for publication
Not applicable.
Copyright
© The Authors 2025.
REFERENCES
1. Sahin TK, Rizzo A, Aksoy S, Guven DC. Prognostic significance of the royal marsden hospital (RMH) score in patients with cancer: a systematic review and meta-analysis. Cancers. 2024;16:1835.
2. Nakamura H, Saji H, Shinmyo T, et al. Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose-uptake in positron emission tomography. Lung Cancer. 2015;87:28-33.
3. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32:3059-68.
4. Jennette JC. Overview of the 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-6.
5. Ricketts CJ, De Cubas AA, Fan H, et al. Cancer Genome Atlas Research Network. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2024;43:113063.
6. Vargová D, Dargaj J, Dohál M, et al. Immune analysis of urine and plasma samples from patients with clear cell renal cell carcinoma. Oncol Lett. 2024;27:281.
7. Takahashi M, Kume H, Koyama K, et al. Preoperative evaluation of renal cell carcinoma by using 18F-FDG PET/CT. Clin Nucl Med. 2015;40:936-40.
8. Betsunoh H, Fukuda T, Anzai N, et al. Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer. 2013;13:509.
9. Betsunoh H, Sakamoto S, Kaji Y, et al. Clinical significance of 18F-fluorodeoxyglucose and glucose transporter 1 mRNA in clear cell renal cell carcinoma. Anticancer Res. 2021;41:5179-88.
10. Kawakami Y, Ohta S, Sayem MA, Tsukamoto N, Yaguchi T. Immune-resistant mechanisms in cancer immunotherapy. Int J Clin Oncol. 2020;25:810-7.
11. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311-35.
12. Nakajima R, Matsuo Y, Kondo T, Abe K, Sakai S. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with renal cell carcinoma. Clin Nucl Med. 2017;42:e177-82.
13. Ito K, Yoshii H, Sato A, et al. Impact of postoperative C-reactive protein level on recurrence and prognosis in patients with N0M0 clear cell renal cell carcinoma. J Urol. 2011;186:430-5.
14. Lee MH, Theodoropoulos J, Huuhtanen J, et al. Immunologic characterization and T cell receptor repertoires of expanded tumor-infiltrating lymphocytes in patients with renal cell carcinoma. Cancer Res Commun. 2023;3:1260-76.
15. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655-63.
16. Sobin LH, Gospodarowicz MK, Wittekind CH. UICC. In: TNM Classification of Malignant Tumors. 7th ed. New York, Wiley-Blackwell 2009; 255-257.
17. Salgado R, Denkert C, Demaria S, et al. International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259-71.
18. Pichler M, Hutterer GC, Stoeckigt C, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer. 2013;108:901-7.
19. Kitajima K, Higuchi T, Fujimoto Y, et al. Relationship between FDG-PET and the immune microenvironment in breast cancer. Eur J Radiol. 2023;158:110661.
20. Zganjar A, Khanna A, Joyce D, et al. Mayo clinic validation of the AUA risk groups for localized renal cell carcinoma. J Urol. 2024;212:331-41.
21. Wang MF, Cai JR, Xia H, Chu XF. Predictive efficacy of the preoperative neutrophil-lymphocyte ratio in lymph node metastasis of cN0 hormone receptor-positive breast cancer. Sci Rep. 2024;14:14216.
22. Yu J, Huang L, Dong T, Cao L. Prediction of outcomes after chemoradiotherapy for cervical cancer by neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. J Obstet Gynaecol. 2024;44:2361858.
23. Mochizuki T, Tsukamoto E, Kuge Y, et al. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551-5.
24. McConkey B, Davies P, Crockson RA, et al. Effects of gold, dapsone, and prednisone on serum C-reactive protein and haptoglobin and the erythrocyte sedimentation rate in rheumatoid arthritis. Ann Rheum Dis. 1979;38:141-4.
25. Shi DY, Xie FZ, Zhai C, Stern JS, Liu Y, Liu SL. The role of cellular oxidative stress in regulating glycolysis energy metabolism in hepatoma cells. Mol Cancer. 2009;8:32.
26. Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143-53.
27. Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1α (HIF-α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361-74.
28. Bacon AL, Harris AL. Hypoxia-inducible factors and hypoxic cell death in tumour physiology. Ann Med. 2004;36:530-9.
29. Chan DA, Sutphin PD, Nguyen P, et al. Targeting GLUT1 and the warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70.
30. Milella M, Rutigliano M, Lasorsa F, et al. The role of MUC1 in renal cell carcinoma. Biomolecules. 2024;14:315.
31. Filippin LI, Vercelino R, Marroni NP, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 2008;152:415-22.
32. Duvigneau JC, Luís A, Gorman AM, et al. Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine. 2019;124:154577.
33. Okada Y, Takahashi A, Ohmiya H, et al. Genome-wide association study for C-reactive protein levels identified pleiotropic associations in the IL6 locus. Hum Mol Genet. 2011;20:1224-31.
34. Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553-72.
35. Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663-71.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data




















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].