The Boston lymphatic center's early experience with lymph node transplantation to the upper extremity
Abstract
Aim: Although vascularized lymph node transplantation (VLNT) has gained recognition as an effective treatment option for lymphedema, no consensus on the timing of transplant with other lymphatic procedures has been established. The aim of this study is to describe our institutional experience with VLNT, including our staged approach and report postoperative outcomes.
Methods: A retrospective review of patients who underwent VLNT for upper extremity lymphedema from May 2017 to April 2022 was conducted. Patients were divided into fat- or fluid-dominant phenotypes based on preoperative workup. Patients with a minimum of 12-month follow-up were included. Records were reviewed for demographic, intraoperative, and surveillance data.
Results: Twenty-three patients underwent VLNT of the upper extremity during the study period, of which eighteen met the study criteria. Nine patients had fluid-dominant disease and nine patients had fat-dominant disease and had undergone prior debulking at our institution. Fluid-dominant patients demonstrated slight reductions in limb volume and hours in compression, and improvement in quality-of-life scores at twelve months. Fat-dominant patients who underwent prior debulking had a slight increase in limb volume without a change in hours of compression, and demonstrated improvements in quality-of-life scores in nearly all subdomains. Overall, 17% of patients discontinued compression therapy entirely. Improvement in extremity edema was present in 83% of postoperative MRIs.
Conclusion: VLNT had varying effects on limb measurements while reliably improving quality-of-life and allowing for the potential of discontinuing compression. Utilizing a staged approach wherein debulking is performed upfront may be particularly beneficial for patients with fat-dominant disease.
Keywords
INTRODUCTION
Upper extremity lymphedema is a debilitating and progressive disease with a substantial impact on patient quality of life[1-4]. Conservative therapies such as decongestive physiotherapy and compression garments are aimed at the palliation of symptoms and prevention of disease progression, but in certain cases, surgical interventions are deemed necessary. An evolving body of evidence demonstrates the beneficial effects of vascularized lymph node transplant (VLNT) on patient quality of life, occurrence of infection, and limb volumes in patients with extremity lymphedema[5-8]. Given its efficacy, VLNT has become a mainstay of treatment for lymphedema and expanding recognition has even led to the creation of a medical policy for insurance coverage for lymphatic surgery, including VLNT[9]. As VLNT has become increasingly adopted by lymphatic centers, programs have developed a staged approach in which VLNT and debulking lipectomy are performed sequentially in efforts to optimize patient outcomes[10-15]. However, because VLNT and debulking greatly differ in their underlying mechanisms and postoperative requirements for compression therapy, the timing and relation of these procedures require careful consideration. To date, a unified consensus has yet to be established on time intervals or the sequence of staged VLNT in relation to debulking lipectomy[16].
Multiple studies have described a staged approach to treat upper extremity lymphedema. Schaverien et al. suggested performing suction-assisted liposuction after physiologic operations to remove excess fatty tissue that VLNT was unable to address[12]. In a similar manner, Nicoli et al. performed laser-assisted liposuction one to three months after VLNT[11]. Similarly, Agko et al. performed liposuction six to eight months after VLNT[13]. Cheng et al. proposed using liposuction after VLNT for patients with lipodystrophy in the proximal limb to decrease the burden of excess fluid on the lymph node flap[10]. Conversely, Cook et al. performed VLNT ten weeks after debulking lipectomy[15]. Similarly, Granzow et al. reported first performing debulking followed by VLNT six to twelve months later to improve functional lymphatic drainage, reduce ongoing fluid accumulation, and decrease the need for compression therapy[14]. Interestingly, these procedures have also been used simultaneously to treat upper extremity lymphedema[17].
At our multi-disciplinary lymphatic center, we have implemented a VLNT program and standardized treatment approach based on patient classification as fat- or fluid-dominant lymphedema phenotype[18-20]. At our center, a debulking lipectomy is consistently performed upfront for patients with a fat-dominant phenotype, followed by a staged VLNT one to two years postoperatively. Patients with a fluid-dominant phenotype are offered VLNT without undergoing a prior debulking procedure. In the current study, we aim to describe our institutional experience with VLNT for the treatment of upper extremity lymphedema and report our postoperative outcomes, including limb volume measurements in the setting of hours of compression therapy per week, radiographic changes, and quality of life. In addition, we describe our management protocol when a combination of VLNT and debulking lipectomy is required for patients with a fat-dominant phenotype.
METHODS
Study design, setting, and population
An observational study was conducted at the Boston Lymphatic Center/Beth Israel Deaconess Medical Center. Institutional review board approval was obtained for this study (Protocol #2022P000092). A review of a prospectively maintained REDCap Quality Improvement Database[21] and a medical review were performed. Patients who underwent vascularized omental lymph node transplant for the treatment of upper extremity lymphedema from May 2017 to April 2022 were identified. Patients were included if they had preoperative measurements, a minimum of 12 months of follow-up, and were treated as per our current algorithm in which patients with fat-dominant diseases underwent debulking lipectomy prior to VLNT. Patient demographics, lymphedema characteristics, intraoperative variables, and surveillance data were extracted for analysis. Baseline characteristics were summarized using means and standard deviations or medians and interquartile ranges (IQR) for continuous data and counts and percentages for categorical data. Descriptive data analysis was performed using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
Preoperative evaluation and identification of surgical candidates
Our center’s approach and evaluation of a patient with lymphedema have been previously described[18]. Determination of lymphedema phenotype (fluid- versus fat-dominant) was performed by an attending radiologist (Tsai LL) as part of our standardized algorithm for evaluation of patients presenting to our center[19,20]. A T2-weighted short-T1 inversion recovery (STIR) image and fat-specific Dixon image were obtained and utilized to grade the proportion of fatty and fluid tissue in the affected limb. Patients with fat-dominant disease who underwent prior debulking were evaluated for VLNT at least one year post debulking lipectomy with stabilized limb volume. Those with a fluid-dominant phenotype were considered for VLNT alone.
Surgical technique
This surgical procedure was performed collaboratively with plastic surgery (Singhal D) and general surgery (Critchlow JF) teams at our institution. Operative notes were reviewed to determine intraoperative details, including the microvascular anastomotic technique.
Intraoperative duplex ultrasonography
An attending radiologist (Tsai LL) performed an intraoperative ultrasound on the back table during the gastroepiploic omental harvest [Figure 1]. The number of lymph nodes within the flap and the overall flap weight were recorded. Our intraoperative duplex ultrasound process for lymph node identification and quantification during VLNT has previously been described in detail[22,23].
Postoperative surveillance
Our standardized process for postoperative surveillance of patients presenting to our Lymphatic Center has previously been described[18,24]. Briefly, during postoperative surveillance visits, limb measurements were obtained by a certified lymphedema physical therapist using perometry and L-Dex (Sozo, Impedimed, Carlsbad, California, USA). Relative volume change (RVC) was calculated using the formula, 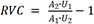 , where A1, U1 are the volume of the affected and unaffected limbs prior to VLNT, and A2, U2 are the volume measurements of the affected and unaffected limbs twelve months post-VLNT[25]. Axial fat-suppressed T2-weighted magnetic resonance imaging (MRI) of the affected extremity was obtained at twelve months after debulking in those with fat-dominant disease, as well as twelve months after VLNT in all patients to assess for changes in subcutaneous edema and confirm lymph node flap viability. All MRI studies were read and interpreted by an attending radiologist (Tsai LL).
, where A1, U1 are the volume of the affected and unaffected limbs prior to VLNT, and A2, U2 are the volume measurements of the affected and unaffected limbs twelve months post-VLNT[25]. Axial fat-suppressed T2-weighted magnetic resonance imaging (MRI) of the affected extremity was obtained at twelve months after debulking in those with fat-dominant disease, as well as twelve months after VLNT in all patients to assess for changes in subcutaneous edema and confirm lymph node flap viability. All MRI studies were read and interpreted by an attending radiologist (Tsai LL).
A validated lymphedema quality-of-life survey (LYMQOL) was administered to patients to assess patient-reported outcomes in four subdomains: appearance, symptoms, mood, and function[26]. Patients were queried regarding the number of hours they spent in compression therapy per week and an interval history of any episodes of cellulitis was obtained, which was defined as an infection of the affected extremity requiring treatment with antibiotics.
RESULTS
During the study period, a total of 23 patients with upper extremity lymphedema were identified, of which 18 met the study inclusion criteria. Five patients were excluded as they had fat-dominant disease and underwent VLNT prior to debulking before we established our current protocol. Of the 18 included, 17 (94%) were female, with a mean age of 57 ± 10 years with a median body mass index of 30 kg/m[2]. Seventeen (94%) were identified as Caucasian and one (5%) as Black or African American. The cohort was stratified by fluid-dominance (n = 9) and fat-dominance (n = 9).
Lymphedema and surgical characteristics were similar among both groups [Table 1]. Of the total cohort, 17 (94%) patients had an oncologic etiology for their lymphedema and one (6%) patient developed lymphedema following an axillary lipoma removal. The time interval from lymphedema diagnosis to the initial surgical consultation appeared to be shorter in the fluid-dominant cohort with a median of 2 (1-4) years compared to the fat-dominant group at 8 (5-15) years. For lymphedema patients with fat-dominant disease, the median time from debulking to VLNT was 18 (16-21) months. For all patients, the median flap weight was 23 (18-28) grams with a median of 6 (5-7) lymph nodes transferred, as identified on intraoperative ultrasound. All lymph node flaps were transferred to the forearm of the affected extremity. Arterial configuration was flow-through[23] in 83% (n = 15) of patients and end-to-side in 17% (n = 3) of patients. Two venous anastomoses were routinely performed on all flaps. On postoperative day three, one patient developed a right upper extremity hematoma at the operative site, requiring urgent evacuation. No other postoperative complications were reported.
Patient demographics, disease characteristics, and VLNT intraoperative variables stratified by lymphedema phenotype
| Overall cohort n = 18 | Fluid-dominant n = 9 | Fat-dominant n = 9 | |
| Baseline characteristics | |||
| Age at VLNT, yrs (mean ± sd) | 57.1 ± 10.1 | 53.4 ± 9.1 | 60.8 ± 10.2 |
| Sex, female (n, %) | 17 (94) | 8 (89) | 9 (100) |
| Race (n, %) | |||
| White | 17 (94) | 8 (89) | 9 (100) |
| Black or African American | 1 (6) | 1 (11) | 0 (0) |
| BMI, kg/m[2] (median, Q1-Q3) | 30.2 (28.8-32.1) | 30.4 (28.8-32.4) | 29.5 (28.5-31.1) |
| Lymphedema characteristics | |||
| Lymphedema laterality (n, %) | |||
| Left side | 8 (44) | 5 (56) | 3 (33) |
| Right side | 10 (56) | 4 (44) | 6 (67) |
| Limb dominance (n, %) | |||
| Left | 2 (11) | 1 (11) | 1 (11) |
| Right | 14 (78) | 7 (78) | 7 (78) |
| Ambidextrous | 2 (11) | 1 (11) | 1 (11) |
| Etiology of lymphedema (n, %) | |||
| Oncologic surgery | 17 (94) | 8 (89) | 9 (100) |
| Non-oncologic surgery | 1 (6) | 1 (11) | 0 (0) |
| Time from lymphedema diagnosis to VLNT evaluation, years (median, Q1-Q3) | 4.2 (2-7.2) | 2 (1-4) | 8 (4.5-15) |
| Time from debulking lipectomy to VLNT, months (median, Q1-Q3) | - | - | 19.4 (16.1-20.3) |
| Surgical characteristics | |||
| Flap weight, grams (median, Q1-Q3) | 23 (17.5-28) | 24 (15-29) | 22 (18.8-28) |
| Recipient location (n, %) | |||
| Forearm | 18 (100) | 9 (100) | 9 (100) |
| Flow-through technique utilized, yes (n, %) | 15 (83) | 6 (67) | 9 (100) |
| No. lymph nodes identified by ultrasound (median, Q1-Q3) | 6 (5-7) | 6 (4-7) | 6 (5-7) |
At twelve months postoperatively, the fluid-dominant group (n = 9) revealed a median limb volume change of -2% (-4% to 2%) with a decrease in hours spent using compression therapy from 47 (1-106) to 4 (0-50) hours per week [Table 2]. This cohort displayed an increase in L-Dex scores from 16 (12-36) to 31 (11-35) and an improvement in all subdomains of LYMQOL at twelve months [Table 3]. Of note, two of the nine patients in this group were able to discontinue compression therapy entirely at twelve months. Of the four patients in this cohort that had postoperative MRI at twelve months, there was a noticeable improvement in edema in 100% (n = 4) of patients, and the lymph node flap was viable in 100% (n = 4) of the studies [Figure 2].
Figure 2. Improvement of upper extremity edema following a vascularized lymph node transplant. Axial fat-suppressed T2-weighted images across the mid right forearm in a patient with right upper extremity lymphedema, pre- (A) and 1-year post-transplant (B) demonstrating interval marked decrease in subcutaneous edema and thickening along the ulnar aspect (*). Arterial-phase post-contrast maximum intensity projection image (C) shows patent flow-through omental artery of the transplant (T). The arrows show signal voids from surgical clips demarcating anastomoses to the ulnar artery (U). R: Radial artery.
Measurements of limb volume and hours spent in compression therapy at the time of preoperative evaluation and 12 months post-VLNT in patients with fat- or fluid-dominant lymphedema phenotypes
| Baseline | 12-month visit | |
| Relative volume change, % | ||
| Fluid dominant (n = 8) | ||
| Median, Q1-Q3 | - | -2% (-4%-1.6%) |
| Fat dominant (n = 9) | ||
| Median, Q1-Q3 | - | 2.6% (-0.4%-5.6%) |
| Compression therapy, hours | ||
| Fluid dominant (n = 6) | ||
| Median, Q1-Q3 | 46.5 (0.8-106) | 3.5 (0-49.8) |
| Fat dominant (n = 9) | ||
| Median, Q1-Q3 | 168 (168-168) | 168 (168-168) |
| L-Dex score | ||
| Fluid dominant (n = 7) | ||
| Median, Q1-Q3 | 15.8 (11.6-36) | 30.6 (10.3-34.8) |
| Fat dominant (n = 8) | ||
| Median, Q1-Q3 | 17 (3.6-25.6) | 16.8 (8.4-25.4) |
LYMQOL Domain Scores at the time of preoperative evaluation and 12 months post-VLNT and at 12 months following VLNT
| Baseline | 12-month visit | |
| Fluid dominant (n = 4) | ||
| Appearance | ||
| Median, Q1-Q3 | 2.6 (1.9-3.3) | 1.8 (1.6-2.1) |
| Functional | ||
| Median, Q1-Q3 | 1.9 (1.6-3.2) | 1.4 (1.4-1.8) |
| Mood | ||
| Median, Q1-Q3 | 2.3 (1.6-3.2) | 1.7 (1.6-1.9) |
| Symptoms | ||
| Median, Q1-Q3 | 2.7 (2.3-2.9) | 2.3 (1.8-2.7) |
| Fat dominant (n = 5) | ||
| Appearance | ||
| Median, Q1-Q3 | 1.4 (1.4-2.4) | 1.4 (1.2-1.6) |
| Functional | ||
| Median, Q1-Q3 | 1.3 (1.2-1.6) | 1.2 (1.1-1.3) |
| Mood | ||
| Median, Q1-Q3 | 1.7 (1.7-1.8) | 1.2 (1.0-1.2) |
| Symptoms | ||
| Median, Q1-Q3 | 2.0 (1.8-2.2) | 1.8 (1.3-2.0) |
Overall, the fat-dominant group (n = 9) demonstrated a limb volume change of 3% (0%-6%) without a change in the overall hours spent in compression therapy. L-Dex scores remained constant at 17 (4-26) preoperatively and 17 (9-25) at twelve months, and an improvement in all subdomains of LYMQOL was noted, except for the appearance subdomain, which remained unchanged. One of the nine patients in this group was able to discontinue compression therapy entirely at twelve months. Of the eight patients in this cohort that had postoperative MRI at twelve months, there was a noticeable improvement in edema in 75% (n = 6) and the lymph node flap was visualized in 88% (n = 7) of images.
The median episodes of cellulitis in the fluid-dominant cohort was 1.25 episodes per year preoperatively and 1.05 episodes per year at twelve months postoperatively. The fat-dominant group had a median of 0.3 episodes per year preoperatively and zero episodes per year in the twelve months following VLNT.
DISCUSSION
In this study, we report our institutional experience and outcomes for omental vascularized lymph node transplant for the treatment of upper extremity lymphedema. Postoperatively, the fluid-dominant cohort demonstrated reductions in both relative limb volume and hours using compression therapy, and had an increase in L-Dex scores at twelve months postoperatively. All LYMQOL subdomain scores improved in this cohort. All patients in this cohort who underwent postoperative imaging revealed an improvement in edema and flap viability on MRI. The fat-dominant cohort had a slight increase in limb volume without an overall change in hours spent using compression therapy or in L-DEX scores, and improvements in quality-of-life scores across almost all subdomains were observed. Of the patients in this cohort who underwent postoperative MRI, 75% displayed an improvement in edema and 88% had confirmed viability of the lymph node flap. Overall, 17% (n = 3) of all patients were able to discontinue compression therapy at twelve months postoperatively.
Previous literature has established that VLNT effectively reduces lymphatic fluid accumulation and potentially eliminates the need for compression therapy; however, VLNT does not address the infiltration of fibroadipose tissue[13,14,27]. In accordance, our study demonstrated improvements in limb volume measures and a reduction in the hours spent in compression therapy in patients with fluid-dominant lymphedema after undergoing VLNT alone. In the fat-dominant cohort, limb volume had a slight increase without a notable change in compression. We have previously reported that fat-dominant patients undergo significant improvements in limb volume following debulking[19]; therefore, we suspect that a ceiling effect may have occurred, indicating that a prior debulking is particularly beneficial for optimizing limb volume in patients with fat-dominant disease. While debulking is targeted at the removal of the fibroadipose tissue, it does not correct the underlying pathophysiologic mechanism of disease, and therefore, patients continue to require lifelong use of compression garments to manage interstitial fluid accumulation[20,28,29]. With longer-term follow-up, we anticipate progressively reducing hours in compression while maintaining optimum limb volume in those debulking patients who underwent staged VLNT. Therefore, we propose the sequence of debulking at least one year prior to VLNT for patients with a fat-dominant phenotype to mitigate disease progression and optimize arm volumes prior to VLNT, as VLNT does not typically result in a total reduction of relative limb volume[8,30,31]. Moreover, we remain concerned that performing debulking after VLNT may put the transplanted lymph node flap at risk and potentially damage newly formed lymphatic networks[32-34].
Adequate compression therapy has a profound impact on limb volume; thus, it is important to present and interpret changes in limb volume in the context of compression use. In the current study, a reduction in the number of hours spent in compression per week was observed in fluid-dominant patients following VLNT, alongside a reduction in limb volume. The overall hours spent in compression for those with fat-dominant disease was unchanged at one year postoperatively, alongside a minimal increase in limb volume. Three patients in the entire cohort did not require any compression therapy after one year. It is particularly important to report changes in limb volume measurements alongside the time patients spend wearing compression garments. Most prior studies that present patient outcomes following VLNT report compression use as a binary variable (either patients are or are not using compression therapy) or as the percentage of patients able to discontinue compression entirely. Quantifying the extent to which patients use compression garments is valuable, as garments can be burdensome in terms of convenience, time expenditure, cost, and comfort. Additionally, patients presenting to our lymphatic surgery clinic often indicate a decrease in disease management as a treatment goal; therefore, delineating the amount of time spent in compression can help determine whether this goal is being met[18]. Additionally, objective measures of limb volume such as RVC and L-Dex can change dramatically over short intervals, so it is useful to interpret these changes in the context of compression garment use. Finally, we note that postoperative changes in limb volume are relatively small in our study. We believe this is closely linked to the fact that our lymphatic surgery program works in tandem with physical therapists in our clinic. Therefore, our patients are already optimized from a limb volume perspective before going to the operating room for VLNT. In this context, hours in compression is an even more important outcome measure.
Improvements in all LYMQOL subdomains were observed in patients with fluid-dominant disease. Similarly, improvements in LYMQOL scores across all subdomains were seen in the fat-dominant group, except for the appearance subdomain, which remained unchanged. The appearance subdomain scores remained relatively constant in patients with fat-dominant disease, possibly because individuals in this cohort likely experienced a dramatic change in their limb volumes following debulking, leading to a major improvement in their perceived appearance that would have occurred prior to VLNT. Overall, the findings from the current study are in concordance with other studies that have reported positive effects of VLNT on patient quality of life[10,35,36]. However, in the current study, the beneficial effects may be less directly related to changes in limb volumes and may be more heavily influenced by the reduction in time spent wearing compression garments. Therefore, assessing patient-centered outcomes such as LYMQOL is imperative in gauging whether treatment goals are being met and assessing the efficacy of VLNT procedures.
Despite minimal changes in limb volume and L-Dex scores among both groups at twelve months post-VLNT, MRI studies obtained at this same time point demonstrated noticeable improvement in edema in 83% (n = 10) and confirmed lymph node flap viability in 92% (n = 11) of patients that underwent postoperative imaging. The radiologic findings in the current study highlight the utility of MRI as an additional modality for measuring subclinical changes in interstitial fluid and additionally underscore the importance of applying a holistic, multi-disciplinary approach for monitoring patients after VLNT. Notably, individual transferred lymph nodes were only visualized on MRI in two patients from the entire cohort, although flap viability was confirmed by MRI in 92%. As the presence and quantity of lymph nodes within the flap were confirmed on intraoperative ultrasound at the time of VLNT, we suspect that our MRIs at the twelve-month time point lack the sensitivity to detect these nodes postoperatively.
A reduction in the median episodes of cellulitis per year was observed in both the fat- and fluid-dominant groups. Only one patient in the fat-dominant cohort had a postoperative case of cellulitis within the twelve months following VLNT, whereas three patients in the fluid-dominant cohort had episodes of cellulitis following VLNT. It is possible that the significantly better outcome that was observed in the fat-dominant patients could be related to the debulking that they previously underwent. This difference may underscore the importance of debulking patients with fat-dominant disease prior to performing VLNT, as debulking targets the removal of fibroadipose tissue, a component that has been established to drive inflammation and clinical progression[37-39]. Mitigation of underlying inflammatory processes is likely related to a decrease in postoperative cellulitis occurrences in patients who underwent prior debulking procedures.
This study is not without limitations. While the vast majority of VLNT procedures utilized a flow-through technique for flap anastomosis, in three patients, this technique was not performed. While we believe the flow-through technique is advantageous for enhancing flap hemodynamics[23], it remains uncertain how other techniques used may affect outcomes. Additionally, as data collection was dependent on patient surveillance visits, certain measures were missing from follow-up. Half the study period occurred as we were initiating our center and the second half occurred during the start of the COVID-19 pandemic, during which lymphatic operations and follow-up visits were frequently canceled or rescheduled. This hindered our ability to obtain a complete dataset. Lastly, the sample size was underpowered and data were analyzed descriptively.
Overall, VLNT had varying effects on limb measurements while reliably improving patient quality of life scores. Importantly, VLNT potentially allows patients to reduce or discontinue compression therapy entirely, and in our overall cohort, three patients were able to achieve this goal at twelve months postoperatively. Furthermore, postoperative radiologic improvement in extremity edema and confirmed flap viability were evident among the vast majority of the cohort. Utilizing a staged approach in which debulking is performed prior to VLNT may be particularly useful in alleviating disease in patients with a fat-dominant phenotype, as both fat and fluid components are targeted. This increases the possibility that a patient in this cohort may reduce or discontinue compression therapy, a result that would not have been achieved from debulking alone. This study provides further evidence for VLNT as an effective treatment for lymphedema and underscores the need for consensus on sequence and timing when staging physiologic and debulking procedures for the treatment of lymphedema.
DECLARATIONS
Authors’ contributionsMade substantial contributions to completion or design of the work: Friedman R, Morgenstern M, Bustos VP, Singhal D, Fleishman A, Tsai LL, Critchlow JF
Performed data acquisition, analysis, interpretation of data for the work: Morgenstern M, Bustos VP, Friedman R, Fleishman A, Singhal D, Shillue K, Tsai LL
All authors helped with drafting or revision of manuscript for important intellectual content and provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Availability of data and materialsData are inputted and stored in a prospectively maintained REDCap Quality Improvement Database.
Financial support and sponsorshipResearch reported in this publication was partially supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (https://www.nhlbi.nih.gov/) under Award Number R01HL157991. Rosie Friedman is sponsored by the 2022 JOBST Lymphatic Research Grant awarded by the Boston Lymphatic Symposium, Inc.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThis research was performed in accordance with the Declaration of Helsinki and approved by the Beth Israel Deaconess Medical Center Institutional Review Board under Protocol #2022P000092.
Consent for publicationConsent was obtained for acquisition of intraoperative patient photographs for research purposes.
Copyright© The Author(s) 2022.
REFERENCES
1. Taghian NR, Miller CL, Jammallo LS, O’Toole J, Skolny MN. Lymphedema following breast cancer treatment and impact on quality of life: a review. Crit Rev Oncol Hematol 2014;92:227-34.
2. Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol 2008;26:5689-96.
3. Chachaj A, Małyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology 2010;19:299-305.
4. Pyszel A, Malyszczak K, Pyszel K, Andrzejak R, Szuba A. Disability, psychological distress and quality of life in breast cancer survivors with arm lymphedema. Lymphology 2006;39:185-192.
5. Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313-5.
6. Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265-75.
7. Cheng MH, Chen SC, Henry SL, Tan BK, Chia-Yu Lin M, Huang JJ. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286-98.
8. Basta MN, Gao LL, Wu LC. Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg 2014;133:905-13.
9. Johnson AR, Otenti D, Bates K, et al. Creating a policy for coverage of lymphatic surgery: addressing a critical unmet need. Plast Reconstr Surg. Forthcoming 2022.
10. Cheng MH, Loh CYY, Lin CY. Outcomes of vascularized lymph node transfer and lymphovenous anastomosis for treatment of primary lymphedema. Plast Reconstr Surg Glob Open 2018;6:e2056.
11. Nicoli F, Constantinides J, Ciudad P, et al. Free lymph node flap transfer and laser-assisted liposuction: a combined technique for the treatment of moderate upper limb lymphedema. Lasers Med Sci 2015;30:1377-85.
12. Schaverien MV, Asaad M, Selber JC, et al. Outcomes of vascularized lymph node transplantation for treatment of lymphedema. J Am Coll Surg 2021;232:982-94.
13. Agko M, Ciudad P, Chen HC. Staged surgical treatment of extremity lymphedema with dual gastroepiploic vascularized lymph node transfers followed by suction-assisted lipectomy-a prospective study. J Surg Oncol 2018;117:1148-56.
14. Granzow JW, Soderberg JM, Dauphine C. A novel two-stage surgical approach to treat chronic lymphedema. Breast J 2014;20:420-2.
15. Cook KH, Park MC, Lee IJ, Lim SY, Jung YS. Vascularized free lymph node flap transfer in advanced lymphedema patient after axillary lymph node dissection. J Breast Cancer 2016;19:92-5.
16. Chang DW, Dayan J, Greene AK, et al. Surgical treatment of lymphedema: a systematic review and meta-analysis of controlled trials. Results of a consensus conference. Plast Reconstr Surg 2021;147:975-93.
17. Leppäpuska IM, Suominen E, Viitanen T, et al. Combined surgical treatment for chronic upper extremity lymphedema patients: simultaneous lymph node transfer and liposuction. Ann Plast Surg 2019;83:308-17.
18. Johnson AR, Fleishman A, Tran BNN, et al. Developing a lymphatic surgery program: a first-year review. Plast Reconstr Surg 2019;144:975e-85e.
19. Granoff MD, Johnson AR, Shillue K, et al. A single institution multi-disciplinary approach to power-assisted liposuction for the management of lymphedema. Ann Surg 2022;276:e613-21.
20. Granoff MD, Pardo J, Singhal D. Power-assisted liposuction: an important tool in the surgical management of lymphedema patients. Lymphat Res Biol 2021;19:20-2.
21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81.
22. Tran BNN, Celestin AR, Lee BT, et al. Quantifying lymph nodes during lymph node transplantation: the role of intraoperative ultrasound. Ann Plast Surg 2018;81:675-8.
23. Johnson AR, Bravo MG, Granoff MD, et al. Flow-through omental flap for vascularized lymph node transfer: a novel surgical approach for delayed lymphatic reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2436.
24. Johnson AR, Fleishman A, Granoff MD, et al. Evaluating the impact of immediate lymphatic reconstruction for the surgical prevention of lymphedema. Plast Reconstr Surg 2021;147:373e-81e.
25. Ancukiewicz M, Miller CL, Skolny MN, et al. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: the flaws in current studies and need for universal methodology. Breast Cancer Res Treat 2012;135:145-52.
26. Keeley V, Crooks S, Locke J, Veigas D, Riches K, Hilliam R. A quality of life measure for limb lymphoedema (LYMQOL). J Lymphoedema 2010;5:26-37.
27. Ito R, Zelken J, Yang CY, Lin CY, Cheng MH. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol Oncol 2016;141:182-8.
28. Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg 1998;102:1058-67.
29. Kung TA, Champaneria MC, Maki JH, Neligan PC. Current concepts in the surgical management of lymphedema. Plast Reconstr Surg 2017;139:1003e-13e.
30. Ciudad P, Agko M, Perez Coca JJ, et al. Comparison of long-term clinical outcomes among different vascularized lymph node transfers: 6-year experience of a single center’s approach to the treatment of lymphedema. J Surg Oncol 2017;116:671-82.
32. Blum KS, Hadamitzky C, Gratz KF, Pabst R. Effects of autotransplanted lymph node fragments on the lymphatic system in the pig model. Breast Cancer Res Treat 2010;120:59-66.
33. Subramanyam P, Janarthanan R, Palaniswamy SS. Early demonstration of spontaneous perinodal lymphangiogenesis by lymphoscintigraphy after vascularized lymph node transplantation - a pilot study. Indian J Nucl Med 2022;37:1-6.
34. Aschen SZ, Farias-Eisner G, Cuzzone DA, et al. Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow. Plast Reconstr Surg 2014;133:301-10.
35. Patel KM, Lin CY, Cheng MH. A prospective evaluation of lymphedema-specific quality-of-life outcomes following vascularized lymph node transfer. Ann Surg Oncol 2015;22:2424-30.
36. Jarvis NR, Torres RA, Avila FR, Forte AJ, Rebecca AM, Teven CM. Vascularized omental lymphatic transplant for upper extremity lymphedema: a systematic review. Cancer Rep (Hoboken) 2021;4:e1370.
37. Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One 2012;7:e49940.
38. Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg 2012;129:825-34.
Cite This Article
How to Cite
Friedman, R.; Morgenstern, M.; Bustos, V. P.; Fleishman, A.; Shillue, K.; Tsai, L. L.; Critchlow, J. F.; Singhal, D. The Boston lymphatic center's early experience with lymph node transplantation to the upper extremity. Plast. Aesthet. Res. 2022, 9, 58. http://dx.doi.org/10.20517/2347-9264.2022.77
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.