Long-term impact of corticosteroid therapy on adult height in juvenile myasthenia gravis patients: a retrospective multicenter cohort study
Abstract
Aim: This study aims to assess the final adult height of patients with juvenile myasthenia gravis (JMG) who received glucocorticoid treatment during childhood.
Methods: A retrospective cohort study was conducted at two neurology centers in Beijing, China, including patients diagnosed with JMG between March 2006 and April 2022. Participants were stratified into two groups: those receiving long-term corticosteroid therapy (≥ 6 months) and those without such treatment. Further subgroup analysis was performed based on treatment timing (prepubertal vs. postpubertal). Mean differences in adult height were calculated, and binary logistic regression was used to evaluate associations between treatment variables and growth outcomes.
Results: Of 120 diagnosed JMG patients, 47 who reached adult height were analyzed. Adult height in the long-term steroid group (168.64 ± 7.58 cm) did not significantly differ from the control group (171.45 ± 9.58 cm)
Conclusion: In JMG patients, steroid treatment after puberty appears to have a relatively smaller impact on adult final height compared to treatment before puberty.
Keywords
INTRODUCTION
Juvenile myasthenia gravis (JMG) is clinically defined as autoimmune neuromuscular junction dysfunction with disease manifestation prior to 18 years of age. This heterogeneous disorder is subclassified into distinct clinical subtypes based on three principal parameters: (1) phenotypic presentation (ocular vs. generalized); (2) thymic pathology (thymomatous vs. non-thymomatous); and (3) serological profiles (anti-acetylcholine receptor antibody [AChR-Ab] seropositive vs. seronegative). Ocular Myasthenia Gravis (OMG) accounts for
Previous cohort studies have consistently shown that early and appropriate use of GC significantly ameliorates clinical symptoms in JMG cases across China, Europe, and the United States. Specifically, GC demonstrates a notable improvement effect in ameliorating extraocular muscle dysfunction[6]. However, it is important to consider the potential adverse effects associated with GC use, including obesity, Cushing’s syndrome, glucose and lipid metabolism abnormalities, and bone metabolism disorders. Cross-sectional studies on adolescent MG highlight GC-induced side effects such as growth retardation, delayed pubertal development, and decreased bone mineral density, all of which substantially impact long-term quality of life[7,8]. The potential sequelae of childhood GC therapy may persist into adulthood, manifesting as reduced final height, chronic metabolic syndrome, and increased fracture risk. While some studies examining the impact of inhaled steroids on the growth and development of children with asthma suggest no effect on final height in adulthood[9], emerging evidence suggests that systemic corticosteroid exposure during critical growth periods may result in permanent height deficits compared to untreated controls[10]. Despite these concerns, GC therapy remains the cornerstone of JMG management in China, though rigorous investigations into its long-term growth effects remain limited. To address this critical knowledge gap, we conducted a retrospective cohort study evaluating final adult height in northern Chinese JMG patients who received childhood GC therapy, aiming to evaluate its influence on growth outcomes.
METHODS
Selection criteria
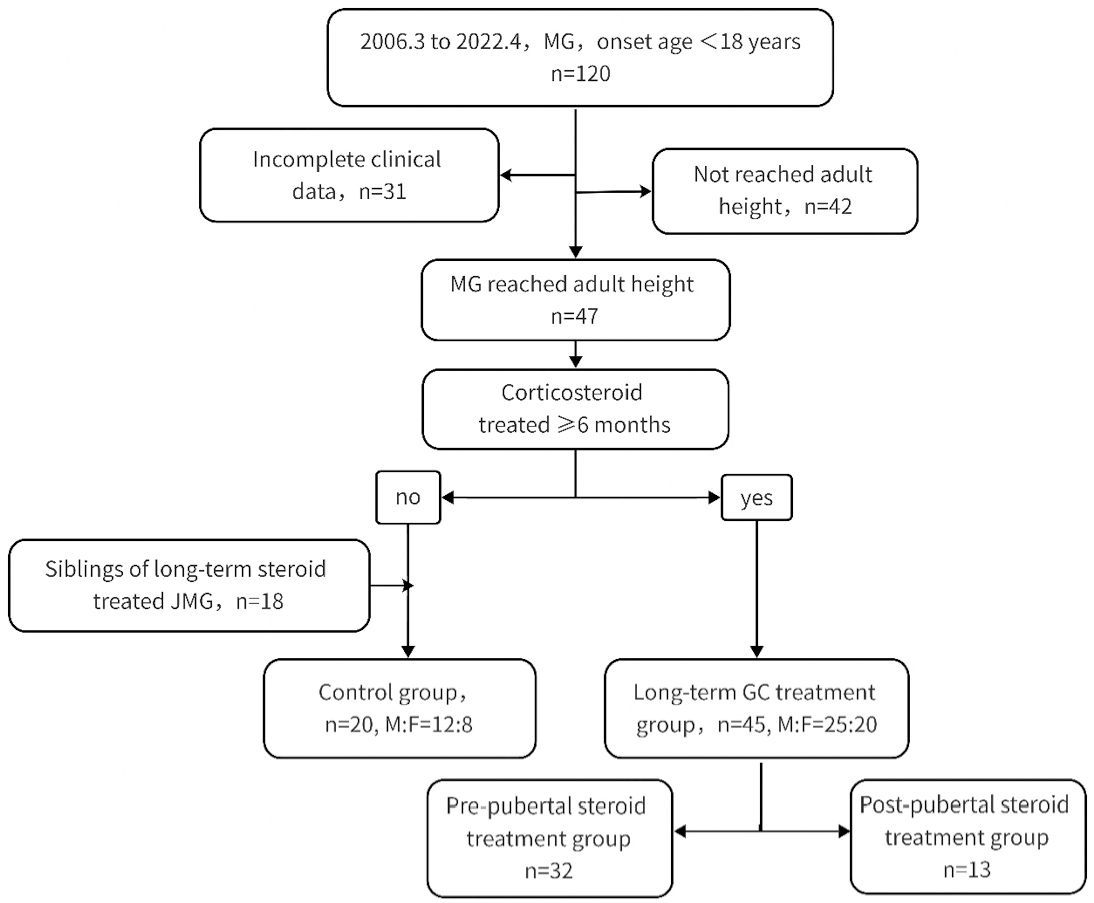
This retrospective study examined medical records of JMG patients from two prominent neurology institutions in Beijing, China, spanning the period from March 2006 to April 2022. Inclusion criteria comprised patients who met the diagnostic criteria of MG and exhibited the onset of MG symptoms before the age of 18. Diagnosis of MG was confirmed based on fluctuating muscle weakness, fatigability, and the fulfillment of neurophysiological and immunological criteria. Exclusion criteria encompassed patients diagnosed with other neuromuscular disorders such as congenital myasthenic syndrome, chronic progressive external ophthalmoplegia (CPEO), or muscular dystrophy. Additionally, individuals with malignant tumors or systemic diseases requiring glucocorticoid (GC) medication were excluded. JMG patients who had not reached their adult height at the time of the last clinical follow-up were also excluded from the study. Participants were stratified into two groups based on the duration of GC therapy: (1) the long-term steroid treatment group (≥ 6 months); and (2) the control group (< 6 months). Siblings of JMG patients without chronic illnesses were included in the control group. Furthermore, JMG patients in the long-term steroid treatment group were subdivided into prepubertal and postpubertal treatment groups, based on the age at which GC therapy was initiated. This stratification aimed to evaluate variations in final adult height across different age groups [Figure 1]. According to recent large-scale epidemiological survey data in China, the median age for the onset of puberty in girls and boys is 9.65 and 10.65 years, respectively[11].
Data collection
Demographic characteristics encompassed patients’ sex, age at last follow-up, final adult height (considered achieved when height increased by less than 0.5 cm for two consecutive years after reaching the age of 15), weight, parents’ height, and siblings’ height. Predicted height was calculated using the following formula: X1 defined as fathers’ height, X2 defined as mothers’ height, for boys, height = 45.99 + 0.78 × [(X1 + X2)/2], and for girls, height = 37.85 + 0.75 × [(X1 + X2)/2][12]. Clinical data were collected through chart review by the research team, including age of GC initiation and maximum daily dose. Disease severity was graded using the Myasthenia Gravis Foundation of America (MGFA) classification system, with clinical changes assessed as post-intervention status (PIS)[13]. Thoracic CT data were gathered and independently reviewed by two experienced physicians. An abnormal thymus was defined as a space-occupying lesion within the anterior mediastinum. For patients who had undergone prior thymic surgery, results of histopathological examination and clinical follow-up data were recorded. Serum samples were analyzed for the presence of antibodies against the AChR (anti-AChR Ab) and muscle-specific kinase (anti-MuSK Ab). The anti-AChR Ab radioimmunoassay (RIA) kit was obtained from IBL International (Hamburg, Germany), while the anti-MuSK Ab RIA kit was sourced from RSR International (Cardiff, UK).
Principle for clinical treatment
All patients diagnosed with JMG received cholinesterase inhibitors (ChEI) as first-line symptomatic treatment. ChEI was administered orally at 3 mg/kg, divided into three times a day. Oral GCs were added for patients failing to achieve symptom control with ChEI monotherapy, with the initial dose adjusted to 0.75-1.0 mg/kg (daily or alternate days), and maintained for 4 weeks following clinical remission. Subsequently, the dose was gradually tapered. In cases that exhibited GC dependency or intolerance, immunosuppressive (IS) agents were incorporated. Tacrolimus (TAC) and azathioprine (AZA) were the most frequently utilized IS agents, and accompanied by routine assessments to monitor potential adverse drug reactions. Patients with a space-occupying lesion revealed by thymic CT underwent thymectomy. Additionally, for JMG patients with inadequate response to oral medications and tested positive for anti-AChR Ab, thymectomy should be considered once they reach adulthood (≥ 18 years of age).
Statistical analysis
Categorical data, such as gender, were categorized using binary classification, while continuous variables like age were presented as continuous variables. Final adult height and predicted height followed a normal distribution and were described using mean ± standard deviation (M ± SD). Non-normally distributed quantitative data, including the differences between final and predicted adult heights, accumulation time of GC, total cumulative dose of GC, and maximum daily dose of GC, were summarized using median with interquartile ranges (25th percentile, 75th percentile). To compare the mean heights between the long-term GC treatment group and the control group, an independent samples t-test was employed. The differences between final-predicted height discrepancies in the two groups were analyzed using the Mann-Whitney U test. The chi-square test was applied to evaluate the disparity in the proportions of individuals achieving their predicted height in the two groups. The correlation between final-predicted height discrepancies and the age of GC initiation, as well as the duration of GC accumulation, was investigated using the Spearman correlation test. Furthermore, binary logistic regression analysis was conducted to identify potential risk factors associated with a final adult height below the predicted height. All statistical tests were carried out using SPSS (IBM SPSS Statistics 20, USA), and a two-sided P-value of < 0.05 was considered statistically significant.
RESULTS
Characteristics of the JMG cohort achieving adult height
A total of 45 JMG patients who received long-term steroid treatment and reached adult height were included in the study, with a male-to-female ratio of 25:20. Symptom onset occurred during childhood
A total of 39 cases underwent testing for AChR-Ab and MuSK-Ab using RIA, with 28 cases (71.79%) tested positive for antibodies. Repetitive nerve stimulation (RNS) tests were performed on 38 cases, with 31 cases (81.58%) demonstrating positive results. For the remaining cases, the diagnosis of MG was confirmed based on pharmacological response to treatment; among the 42 patients who completed the neostigmine test, 35 cases (83.33%) demonstrated a positive result. Thymectomy was performed in 17 cases (37.78%), none of whom had thymoma. Additionally, 14 cases (31.11%) were diagnosed with concomitant hyperthyroidism. In 13 cases (28.88%), spontaneous improvement without any therapeutic intervention was observed. The median duration of GC treatment was 3 years (1.4, 5.85), with the highest daily oral dose of prednisone equivalent reaching a median of 55 mg (25, 70). Within this cohort, 38 individuals (84.44%) achieved minimal manifestation status (MMS) or better at the time of follow-up.
Height discrepancies between long-term steroid-treated JMGs and controls
In the control group, which consisted of non-long-term GC-treated JMG patients and their siblings (n = 20, male: female = 12:8), 60% of individuals (12/20) achieved their predicted adult height. There were no significant differences between the two groups in terms of age (P = 0.23) and gender distribution (P = 0.79). Comparative analysis revealed no significant differences between groups in either predicted adult height (169.67 ± 6.94 vs. 170.16 ± 6.85 cm, P = 0.79) or final adult height (168.64 ± 7.58 vs.
Demographic and clinical characteristics of two groups
| Characteristics | Long-term steroid treatment JMGs | Control | Comparison | |
| n = 45 | n = 20 | Z/χ2/t | P | |
| Gender, [female, (n, 9%)] | 20 (44.44) | 8 (40) | χ2 = 9.93 | 0.79 |
| Age, [years, M (P25, P75)] | 22.19 (19, 25.5) | 20 (19.25, 23.75) | Z = -1.04 | 0.23 |
| Duration of GC therapy, [year, M (P25, P75)] | 3 (1.4, 5.85) | NA | NA | NA |
| Target adult height, [M ± SD, cm] | 169.67 ± 6.94 | 170.16 ± 6.85 | t = 0.26 | 0.79 |
| Final height, [M ± SD, cm] | 168.64 ± 7.58 | 171.45 ± 9.58 | t = 1.27 | 0.26 |
| ΔFinal height-target height, [Mean, 95%CI] | -1.03 (-2.13, 0.08) | 1.30 (-1.14, 3.74) | Z = -1.84 | 0.06 |
| PIS, n (%) | NA | |||
| MMS or better | 38 (84.44) | 2 (100) | ||
| Improved | 6 (13.33) | 0 | ||
| Unchanged or worse | 1 (2.22) | 0 | ||
| Muscle involvement at onset#, n (%) | NA | |||
| Extraocular muscle | 22 (70.97) | 2 (100) | ||
| Appendicular muscle | 9 (29.03) | 0 | ||
| MG type at nadir, n (%) | NA | |||
| OMG | 33 (73.33) | 2 (100) | ||
| GMG | 12 (26.67) | 0 | ||
| Age at prednisone initiation, n (%) | NA | |||
| Prepuberty | 32 (71.11) | 0 | ||
| After puberty | 13 (28.89) | 0 | ||
Factors influencing the final height in JMGs undergoing long-term GC treatment
Factors affecting final height and final-predicted height discrepancies
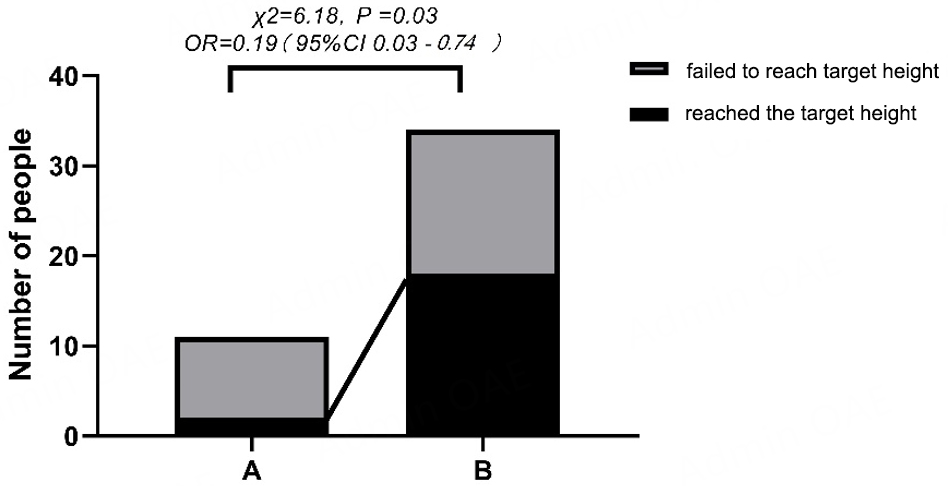
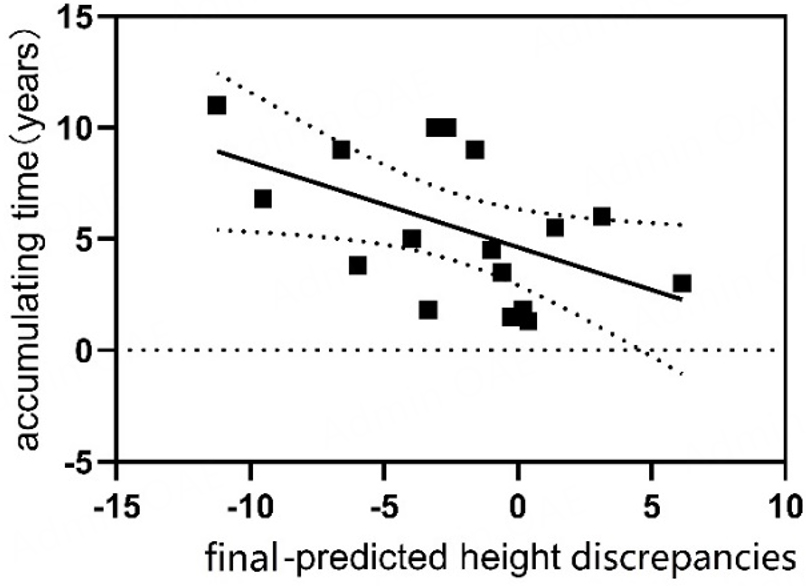
Thirteen individuals began GC treatment before puberty, with 84.6% (11/13) failing to achieve their predicted adult height. In contrast, among 32 patients who initiated GC after puberty, 43.8% (14/32) did not reach their predicted height, this difference was statistically significant (χ2 = 6.18, P = 0.03), with prepubertal GC initiation associated with a substantially increased risk of failing to achieve predicted height (OR = 0.19, 95%CI: 0.03 to 0.74) [Figure 2]. Patients who initiated glucocorticoid (GC) treatment before puberty demonstrated a mean final-predicted height discrepancy of -2.55 cm (95%CI: -4.62 to -0.48), significantly greater than the -0.41 cm (95%CI: -1.72 to 0.91) observed in patients who began treatment after puberty (median difference: -2.86 vs. 0.19; Z = -2.12, P = 0.03). Correlation analyses identified significant associations between height discrepancies and treatment parameters. The age at GC initiation showed a positive correlation with final height outcomes (r = 0.23, P = 0.04), while cumulative GC duration demonstrated a negative correlation (r = -3.11, P = 0.04) [Figure 3]. Subgroup analyses revealed no statistically significant differences in baseline characteristics between patients who achieved their predicted height and those who did not [Supplementary Table 1]; however, non-parametric comparisons revealed significant differences in maximum daily steroid dosage between groups (50 [37.5-60] vs. 60 [56.25-70] mg; Z = -2.64, P = 0.008). Notably, total cumulative dosage showed no significant variation (14,227.5
Figure 2. Proportions of individuals reaching predicted height in two groups (GC initiation before puberty vs. after puberty). A: JMG started corticosteroid therapy before puberty, contained a higher proportion of individuals not reaching predicted height; B: JMGs started corticosteroid therapy after puberty.
Factors associated with failure to achieve predicted final height
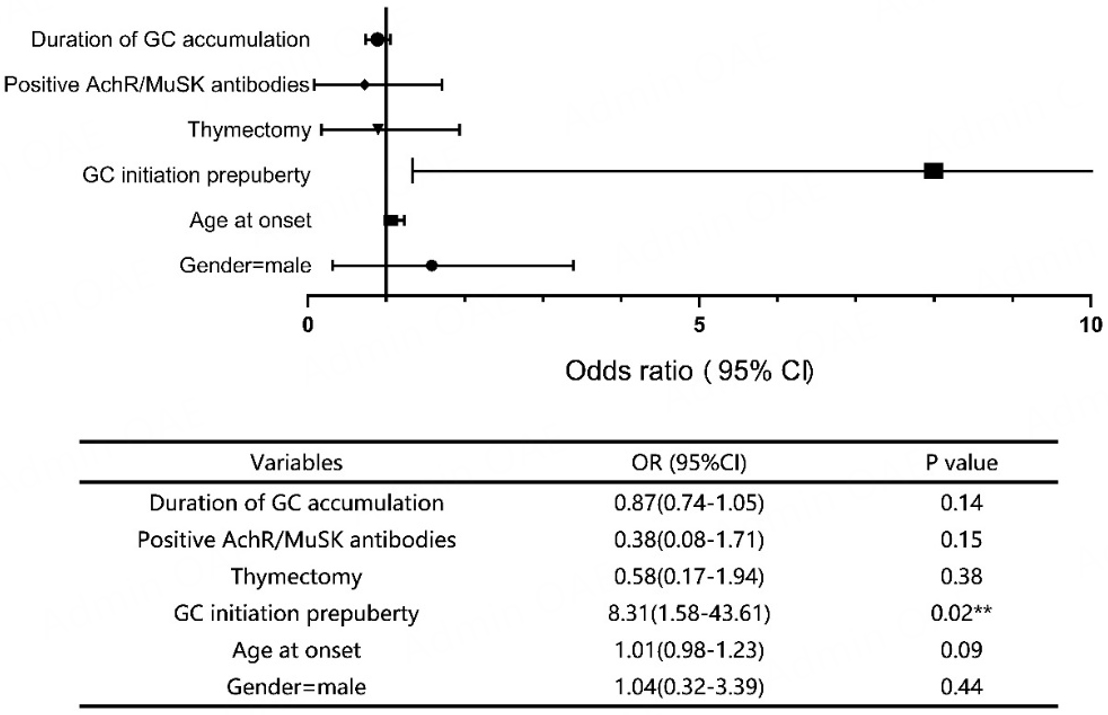
The risk factors associated with a final height below the predicted value were analyzed using univariate logistic regression, as illustrated in Figure 4. Multivariate logistic regression was subsequently performed to identify independent predictors, incorporating the following variables: initiation of GC treatment before puberty, cumulative duration of GC treatment and positive anti-AChR or anti-MuSK antibodies. After adjustment for age and gender, initiation of GC treatment before puberty was identified as an independent risk factor for failure to achieve predicted height (OR = 11.07, 95%CI: 1.24-99.15, P = 0.03).
DISCUSSION
This study provides valuable insights into the long-term impacts of GC treatment on growth outcomes in patients with JMG. In this retrospective analysis, participants were stratified into two groups based on long-term GC treatment exposure: a long-term GC treatment group and a control group consisting of JMG patients without long-term GC treatment and their siblings. The two groups were well-matched in terms of age, gender distribution, and predicted height.
Upon comparing the GC treatment JMG group with the control group, no significant statistical differences were found in key growth parameters, including final adult height, the proportion of individuals achieving predicted height, or the discrepancies between final and predicted height. Thus, based on the existing data, long-term GC treatment does not appear to adversely affect final adult height in JMG patients. This conclusion aligns with previous research, notably a study involving asthmatic children, which demonstrated that steroid-treated individuals could attain their expected height[9], highlighting new theoretical evidence to the understanding of long-term growth outcomes in JMG patients undergoing GC treatment.
In previous research on JMG, the majority of investigations have adopted a cross-sectional approach. A study revealed lower bone density in JMG patients compared to their healthy counterparts, alongside decreased bone age and reduced vitamin D activity[12]. In the present study, a detailed analysis of growth outcomes revealed that 60% (12/20) of individuals in the control group achieved their predicted height, whereas only 44.4% (20/45) of GC-treated JMG patients attained their predicted height. Although this difference did not reach statistical significance, a crucial finding emerged: the mean discrepancies between final and predicted height were negative in the GC treatment group, contrasting with a positive mean discrepancy in the control group. This finding suggests the potential intricacies in the relationship between GC treatment and height outcomes, especially in chronic conditions like JMG.
To elucidate the determinants of final height outcomes, this study conducted comprehensive correlation analyses. The results unveiled linear correlations between the age of initiating GC treatment and the discrepancies between final and predicted height (r = 0.23, P = 0.04). Additionally, the duration of GC treatment accumulation was correlated with final adult height discrepancy (r = -3.1, P = 0.04). These findings highlight the critical importance of optimizing the duration of GC treatment, particularly in prepubertal children, to mitigate potential adverse effects on growth outcomes. Subgroup analyses stratified by gender, antibody type (AChR vs. MuSK), and thymectomy status demonstrated no significant differences in the proportion of individuals failing to achieve their predicted height (P > 0.05 for all comparisons).
Our analysis revealed that individuals who initiated GC before puberty exhibited a propensity for negative discrepancies between final and predicted height, with a significantly higher proportion failing to achieve their predicted height. Binary logistic regression analysis identified prepubertal initiation of GC treatment as the sole independent risk factor for failing to attain predicted height (OR = 8.31, 95%CI: 1.58-43.61,
The study cohort exhibited a balanced gender distribution, with the majority of JMG cases (53.33%, 24/45) presenting during childhood (< 14 years of age). Ocular myasthenia constituted 73.33% of cases at nadir, while generalized myasthenia accounted for 26.67%. The conversion rate from ocular to generalized myasthenia was 25.53%. Throughout adolescence, ocular symptoms remained the predominant manifestation in most JMG cases, often inadequately controlled by acetylcholinesterase inhibitors alone. This highlights the critical need to explore safe and effective non-GC therapeutic options, particularly for prepubertal patients, to manage MG-related ocular symptoms. At the final follow-up, 20 cases achieved Status-Minimal Manifestation (PIS-MM), 12 cases reached Pharmacologic Remission (PR), and 6 cases achieved Complete Stable Remission (CSR). The combined proportion of patients achieving PIS-MM or better outcomes was (38/45, 84.44%). This percentage exceeded those reported in prior related studies, likely attributable to the early initiation and appropriate dosage of GC treatment in this cohort. Notably, 28.88% of patients experienced spontaneous symptom remission without the need for pharmacological interventions after disease onset, further underscoring the heterogeneous nature of JMG progression and treatment response.
Certainly, there are several limitations to acknowledge in this study. Firstly, the retrospective study design introduces inherent biases that cannot be overlooked. Secondly, disparities emerge in age at onset and age at GC initiation between JMG patients who have reached adult height and those who have not. This imbalance is attributed to challenges in follow-up, with a notable number of participants lost to follow-up, which is inevitable in retrospective studies. Thirdly, this article lacks continuous records of patients’ weight and height, as well as standardized measurement tools for adult height. This issue warrants careful consideration in actual clinical practice, emphasizing the need for prospective studies to offer more objective conclusions regarding JMG patients from China.
In recent years, non-corticosteroid immunosuppressive agents, such as tacrolimus, have gained increasing utilization among Chinese JMG patients. Furthermore, the potential application of biological targeted drugs like eculizumab and rituximab in adolescent populations represents a promising avenue for future treatment strategies. These advancements in immunotherapy underscore the need for continued investigation into the long-term effects of GC on adult height in JMG patients within the context of evolving therapeutic landscapes.
DECLARATIONS
Authors’ contributions
Conceptualization: Tan Y, Xie Q, Huang Y, Yan J, Shi J, Li M, Zhang Y, Guan Y, Jing Y
Original draft preparation, Tan Y, Xie Q
Review and editing: Guan Y, Jing Y
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available from the corresponding authors upon reasonable request.
Financial support and sponsorship
This work was supported by the National High Level Hospital Clinical Research Funding (grant number: 2022-PUMCH-B-017).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The protocol received approval from the Research Ethics Committee of Clinical Research of Peking Union Medical College Hospital, Beijing, China and was conducted in agreement with the principles of the Declaration of Helsinki and local ethical standards, Number: K22C2029. Parents/guardians of study participants provided written informed consent.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Yang L, Tang Y, He F, et al. Clinical characteristics and outcome predictors of a Chinese childhood-onset myasthenia gravis cohort. Front Pediatr. 2022;10:996213.
2. Huang X, Li Y, Feng H, Chen P, Liu W. Clinical characteristics of juvenile myasthenia gravis in Southern China. Front Neurol. 2018;9:77.
3. Vecchio D, Ramdas S, Munot P, et al. Paediatric myasthenia gravis: prognostic factors for drug free remission. Neuromuscul Disord. 2020;30:120-7.
4. Sugimoto T, Ochi K, Ishikawa R, et al. Initial deterioration and intravenous methylprednisolone therapy in patients with myasthenia gravis. J Neurol Sci. 2020;412:116740.
5. Barraud C, Desguerre I, Barnerias C, Gitiaux C, Boulay C, Chabrol B. Clinical features and evolution of juvenile myasthenia gravis in a French cohort. Muscle Nerve. 2018;57:603-9.
6. Europa TA, Nel M, Heckmann JM. Myasthenic ophthalmoparesis: time to resolution after initiating immune therapies. Muscle Nerve. 2018;58:542-9.
7. Braz NFT, Rocha NP, Vieira ÉLM, et al. Negative impact of high cumulative glucocorticoid dose on bone metabolism of patients with myasthenia gravis. Neurol Sci. 2017;38:1405-13.
8. Finnis MF, Jayawant S. Juvenile myasthenia gravis: a paediatric perspective. Autoimmune Dis. 2011;2011:404101.
9. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-9.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-12.
11. Pu JQ, Zhang JW, Chen RM, et al. Survey of height and weight of children and adolescents at different Tanner stages in urban China. Zhonghua Er Ke Za Zhi. 2021;59:1065-73.
12. Luo ZC, Low LCK, Karlberg J. A comparison of target height estimated and final height attained between Swedish and Hong Kong Chinese children. Acta Paediatr. 1999;88:248-52.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].