Exploratory survey study on adjunctive use of medical cannabis for Gaucher disease
Abstract
Aims: Using an investigator-designed survey tool to confirm that adult patients with type 1 Gaucher disease (GD1) often self-prescribe cannabis products to try to alleviate symptoms such as lingering fatigue, chronic bone and joint pain, loss of energy, anxiety, and depression that persist despite enzyme replacement therapy (ERT) or substrate restriction therapy (SRT). Additionally, to explore whether patient reports of symptom relief and adverse side effects relate to frequency and duration of cannabis use.
Methods: We conducted an anonymous, cross-sectional questionnaire study to elicit GD1 patient-reported experiences with cannabis used to alleviate symptoms they attributed to their underlying disease. Eligible participants included individuals with GD1 aged ≥ 18 years, regardless of sex, gender, country of residence, ethnicity, state of health or GD1 treatment status. The questions included basic socio-demography (n = 9), GD diagnosis and pre-treatment signs and symptoms (n = 16), GD treatment information (n = 9), current GD symptoms (n = 12), concurrent manifestations of Parkinson’s disease (n = 6), details of cannabis use (n = 24), perceived effect of cannabis on symptoms (n = 13), and interest in participating in future studies (n = 2).
Results: 159 GD1 adults (81.5% US) responded to advertisements on patient online sites. The most frequent pre-treatment symptoms were fatigue (83.8%), bone or joint pain (79.7%), and bleeding problems (73.0%). Hemostasis substantially improved, but pain, achiness, fatigue, and anxiety often persisted. Sixty-two respondents (39%) reported very heterogeneous cannabis use. There was a positive association between the severity of persistent symptoms and the likelihood of cannabis use. Cannabis users reported improvements in muscle pain (84.3%), bone pain (82.4%), joint pain (82.4%), anxiety (70.6%), and general achiness (66.7%). However, moderate and extreme bone manifestations, fatigue, breathing problems, memory loss, and episodic dyscoordination were more prevalent among frequent users of inhaled cannabis than among non-users.
Conclusion: Our results justify further investigations to determine the efficacy and safety of cannabis specifically for GD1 patients. Although randomized controlled trials would be optimal, well-designed observational GD registry studies may be a more practical approach. Although some patients may be reluctant to talk openly with their doctors about cannabis, they should routinely be queried about such use by primary care physicians and GD specialists who, in turn, must be able to provide informed guidance on safety, dosage, and potential interactions with other medications the patient is using.
Keywords
Correction published on 7 November 2024, see Rare Dis Orphan Drugs J 2024;3:32.
INTRODUCTION
Gaucher disease (GD), the most common autosomal recessively transmitted LSD, has an estimated global incidence of 1.5-2.5 per 100,000 live births[1,2]. The neuronopathic variants (GD2 and GD3) are characterized by primary degenerative neurologic disease, severe systemic manifestations, and mortality between infancy and early adulthood[3]. Type 1 GD (GD1) is not associated with clinically evident central nervous system disease, with the exception of 5%-10% of patients who develop Parkinson-like neurodegeneration, most often after the seventh life decade[4]. The prognosis in untreated patients with GD1 is highly variable, encompassing a minority of individuals, largely of Ashkenazi Jewish descent, with minimal signs and symptoms at diagnosis, little evidence for progression, and normal life expectancy, and a majority with variably severe acute and chronic morbidities associated with hepatosplenomegaly, anemia, thrombocytopenia, bleeding diatheses, and especially skeletal pathologies including bone mineral loss, decreased bone strength and integrity, focal osteolysis, propensity for fragility fractures, bone marrow infarction, cortical osteonecrosis, and joint collapse[5]. Besides GBA1-associated Parkinson’s disease, other late complications include increased risk for gammopathies, myeloma, lymphoma, cirrhosis, and hepatocellular carcinoma[6]. Common constitutional symptoms such as fatigue, chronic pain, and asthenia often translate to economic, psychosocial, and spiritual suffering, decreased quality of life, opioid addiction, loss of hope, and even increased risk for suicide[7,8].
The genetics, biochemistry, and pathophysiology of GD are well, if yet incompletely, understood and the current state of knowledge is amply reviewed elsewhere[5,7]. For the purposes of this report, it is sufficient to indicate that GD is caused by in trans pathogenic variants in GBA1 located on chromosome 1q21. GBA1 codes the biosynthesis of acid-β-glucocerebrosidase (GCase), a lysosomal hydrolase that catalyzes glycolysis of glucosylceramide (GL1) to ceramide and glucose. Attenuated GCase activity coupled with secondary upregulation of GL1 synthase results in lysosomal accumulation of the primary substrate GL1 and pathogenic secondary substrates such as glucosylsphingosine[7], a process that ultimately fuels many clinical manifestations of GD, but that also leads to the development of intravenous enzyme replacement therapy with pharmacologic recombinant glucocerebrosidases (ERT) and, subsequently, oral substrate restriction therapy (SRT) with small molecule inhibitors of GL1 synthase[9]. Each treatment by itself alleviates many systemic GD1 manifestations, including hepatosplenomegaly, anemia, thrombocytopenia, and bone crises[9,10]. However, neither is curative. Many patients who suffered irreversible skeletal and joint complications because of delayed diagnosis and treatment inception, continue to be symptomatic even after reversible disease manifestations have been achieved, and most treated patients have some evidence of residual disease indicated by low-level increases in biomarkers such as plasma lyso-GL1 and chitotriosidase, persistent splenomegaly and bone marrow infiltration, and unresolved osteopenia[11-13].
Therefore, there is a continuing need for adjunctive therapies ranging from orthopedic interventions to physical and occupational therapy and pharmacologic and non-pharmacologic interventions[14]. Nonsteroidal anti-inflammatory medications, anxiolytics, and anti-depressants that are the mainstays of these adjunctive regimens have limited efficacy, and all have potentially deleterious side effects, especially in older people. As with many other individuals with chronic pain, the use of opioids in patients with GD is associated with a risk for side effects and addiction and should be discouraged[15,16]. Anecdotal evidence suggests that some GD1 patients use cannabis to manage these persisting symptoms, sometimes without their physicians’ knowledge.
Cannabis sativa L. contains more than 100 known chemical compounds classified as phytocannabinoids and has been used medicinally and therapeutically for millennia[17-19]. D9 tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most extensively studied[17]. Medicinal benefits of cannabis are generally attributed to anti-inflammatory properties mediated through agonistic activation of the endocannabinoid system (ECS) which is comprised of endogenous cannabinoids, cannabinoid receptors, and the enzymes responsible for the synthesis and degradation of endocannabinoids[17,18]. A study of 5,363 adults found decreased levels of systemic inflammation biomarkers (hsCRP, IL-6, and fibrinogen) 30 days following cannabis use[20-22]. CBD has been shown to possess analgesic, anti-inflammatory, and antioxidant properties[23-26]. With potential relevance to GD, there are studies linking the ECS to bone metabolism, homeostasis, and healing[27,28].
Pharmacologic effects of exogenous, mixed composition phytocannabinoids can be complex as they interact with the endocannabinoid system of receptors, endogenous ligands and associated synthetic and degradative enzymes with potential applications for energy balance, appetite stimulation, blood pressure regulation, pain modulation, embryogenesis, nausea and vomiting control, memory, learning, and immune responses[29].
Although, to date, Epidiolex for epilepsy is the only phytocannabinoid drug with FDA approval (in addition to three synthetic THC-related drugs largely used as anti-emetics), medicinal usage of cannabis, including products with high THC content, is legal in most US states for the treatment of cancer, epilepsy, multiple sclerosis, glaucoma, HIV/AIDS, Parkinson's disease, Crohn's disease, PTSD, ALS, terminal illnesses, and chronic nonmalignant pain, and in children with severe neurological illnesses including cerebral palsy, muscular dystrophy, cystic fibrosis, and osteogenesis imperfecta[30]. In many countries other than the US, the use of medicinal cannabis is not authorized and even criminalized.
In randomized controlled trials (RCT) involving patients with multiple sclerosis, post-trauma and post-surgical neuropathic pain, ulcerative colitis, and schizophrenia, various cannabis products effectively alleviated symptoms such as spasticity, central and peripheral neuropathy, sleep disturbance, abdominal pain, diarrhea, and psychotic ideation[31-35]. Regarding benefits for chronic non-cancer pain, meta-analyses were inconclusive[36,37]. A patient-reported outcome (PRO) study using cannabis products with defined THC-CBD concentrations in patients primarily with non-cancer chronic pain reported clinically significant improvements in multiple SF-36 QOL domain scores[38]. Herbal cannabis use over 5 months resulted in significant pain reduction and decreased opioid use in elderly patients with cancer-associated pain[39]. Adverse, largely non-serious effects of cannabis, mostly associated with high THC formulations, include headache, dry eyes, burning sensation in areas of neuropathic pain, dizziness, drowsiness, numbness, cough, psychotropic effects, and potential for drug dependency[26,31,33,36,38-41]. A meta-analysis suggests that CBD has relatively few serious adverse effects, but that potential adverse interactions with other medications should be monitored carefully[42].
To the best of our knowledge, cannabis use in patients with GD has not been studied. As a preliminary step toward researching the potential of cannabis as adjunctive therapy in the management of GD symptoms that persist despite ERT/SRT, we created and disseminated a survey among GD1 patients to assess patient-reported GD history, current GD status, history of cannabis use, if any, and perceived therapeutic benefits or adverse effects.
METHODS
We conducted an anonymous, international, cross-sectional questionnaire study between December 2020 and February 2021 designed to elicit GD1 patient-reported experiences with the use of cannabis, whether medicinal or recreational, to alleviate symptoms they attributed to their underlying disease (Study protocol and survey questionnaire, Supplementary Material). Eligible participants included adult
Interested parties were directed to an online explanatory disclosure document (see protocol in Supplementary Material) that included a link to the survey. All individuals who accessed the link received a $5.00 Starbucks e-gift card (or equivalent thereof) regardless of survey participation. Completion of the survey was indicative of voluntary consent, as explained in the disclosure document and survey introduction. To preserve anonymity, the email addresses of participants (needed for gift card distribution) were not retained. Access to the data was restricted to the principal investigators and their research assistants.
The primary objective of this study is to confirm our impression that adult patients with GD1, particularly those with moderate to severe persistent symptoms, have historically used cannabis products to alleviate those symptoms. Secondarily, we wished to probe to what extent non-validated patient-reported outcomes (symptom relief and adverse side effects) were related to the frequency and duration of cannabis use. The survey questions include basic socio-demography (n = 9), GD diagnosis and pre-treatment signs and symptoms (n = 16), GD treatment information (n = 9), current GD symptoms (n = 12), concurrent manifestations of PD (n = 6), details of cannabis use (n = 24), perceived effect of cannabis on symptoms
The collected data were imported to SPSS version 28, where they were cleaned, and descriptive statistics such as frequencies, percentages, means, standard deviations, medians, and interquartile ranges were calculated. The prevalence of cannabis use was estimated, and its 95% confidence interval was computed. The influences of ERT on GD symptoms were assessed using the chi-square test. The chi-square test was also used to evaluate the association between cannabis use and current symptoms of GD. In all cases,
RESULTS
Because of the indirect recruitment methods described above, the number of potential participants is unknown, and a response rate cannot be calculated. A minimum of 970 adult patients with GD were directly contacted through one Facebook group and an indeterminate number up to another 900 (with possible duplicates) were contacted in other Facebook groups. In addition, an unknown number of recruitment flyers were electronically forwarded by GD patients to other patients with whom they maintained contact via a listserv. 159 individuals submitted a complete survey questionnaire. 71% were female (n = 114), just over 50% were Jewish (n = 80), and 81.5% (n = 129) were from the United States of America (US). The median age was 45 years (19-80) and the median age at GD diagnosis was 12.5 years [Supplementary Table 1]. 145 respondents (91.2%) reported ongoing treatment with ERT or SRT. A majority were currently treated with either imiglucerase, velaglucerase alfa, or eliglustat. Seventy-eight patients were treated for more than 20 years. Of the 49 patients who reported that they are currently using eliglustat, nine had been on treatment for less than 10 years. Forty of 49 patients currently using eliglustat previously had ERT. Except for the over-representation of women and the younger-than-expected median age at diagnosis, our cohort is anthropometrically similar to larger US study cohorts in publications of the International Collaborative Gaucher Group (ICGG) Registry
Because of the known association of GBA1 mutations with an increased risk of developing Parkinson’s disease (PD), we included questions regarding PD diagnosis or symptoms. Although no respondent reported a PD diagnosis, 15 answered “I am not sure”. Compared to the 145 GD patients who denied any diagnosis of PD, the 15 “unsure” individuals (median age 41 years [32y-69y]) reported a disproportionately high prevalence of memory loss and dyscoordination ranging from mild to extreme severity (memory loss: 73.3% vs. 39%; dyscoordination: 53.3% vs. 19.3%). Cannabis use was neither more nor less prevalent among these individuals than among the other 144 survey participants.
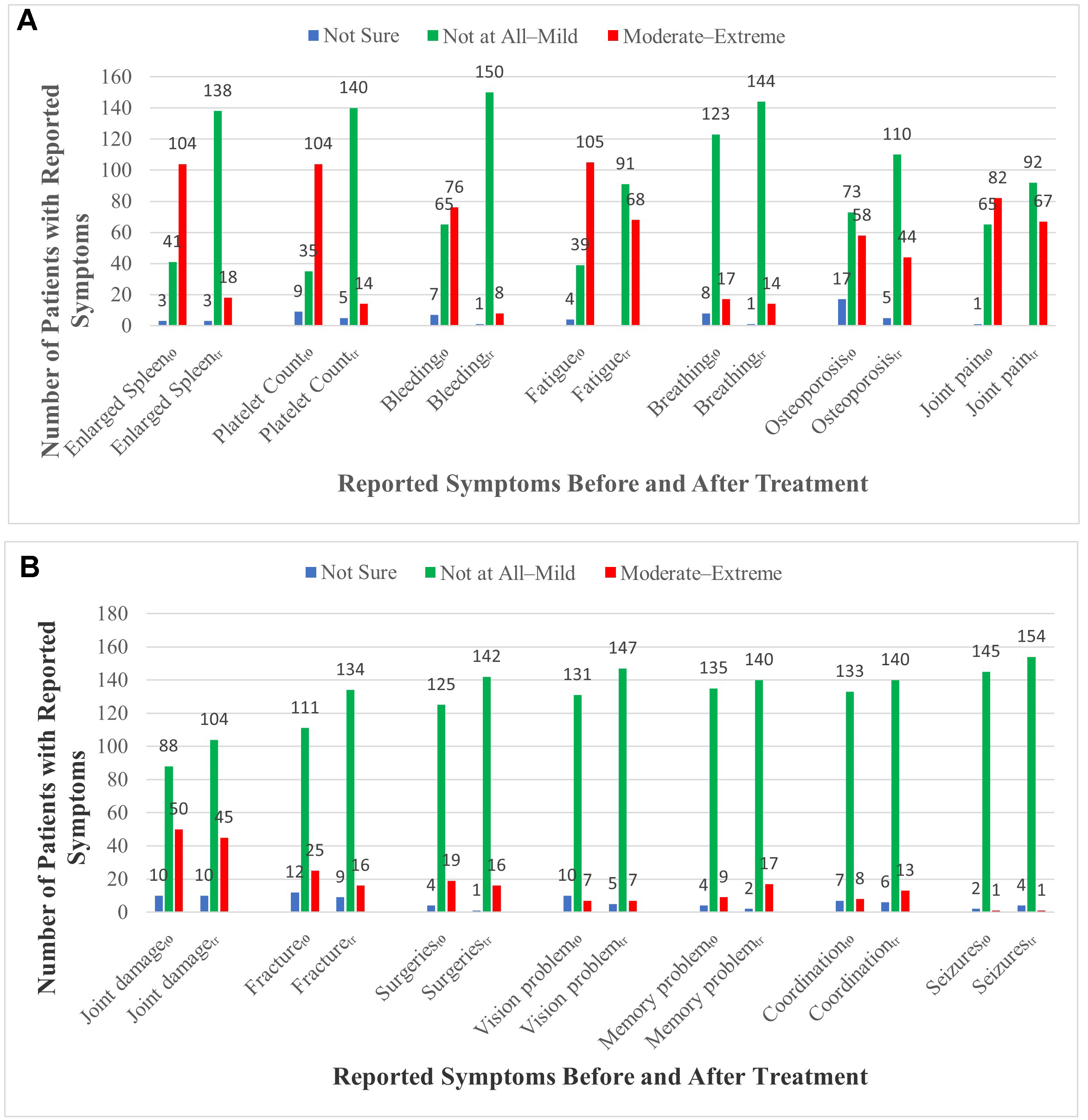
Because our survey was designed to capture patient-reported outcomes, we utilized easy-to-understand symptom descriptions, and we presented five severity rankings: not sure, not at all, mild, moderate, and extreme. The effects of ERT/SRT on the designated GD manifestations for all severity categories are shown in Figure 1 and Supplementary Table 2. The pre-treatment “baseline” data are, for a large majority of patients, based on recall of signs and symptoms more than 10-20 years in the past. We do not know if survey respondents referenced old records rather than memory. Before starting GD treatment, 104/159 patients (64%) reported having moderate-extreme splenomegaly and thrombocytopenia, with 76 (48%) reporting bleeding episodes. These manifestations were markedly reduced post-treatment (splenomegaly 18/159 [11%]; thrombocytopenia 14 [9%]; bleeding 8 [5%], with approximately half of these patients having been treated for less than 5 years). However, moderate-extreme fatigue, bone pain, joint pain associated with joint damage, and reduced bone mineral density continued to be present in approximately 40% of patients despite years of ERT/SRT.
Figure 1. Patient-reported effects of disease-specific treatment (ERT/SRT) on common GD signs and symptoms. T0 refers to the signs and symptoms reported by the respondents at the time that GD-specific treatment (ERT or SRT) was initiated. Tr refers to response data on the dates of completion of the questionnaires (DEC 2020 - FEB 2021). (A) Change in symptoms 1-7; (B) Change in symptoms 8-14.
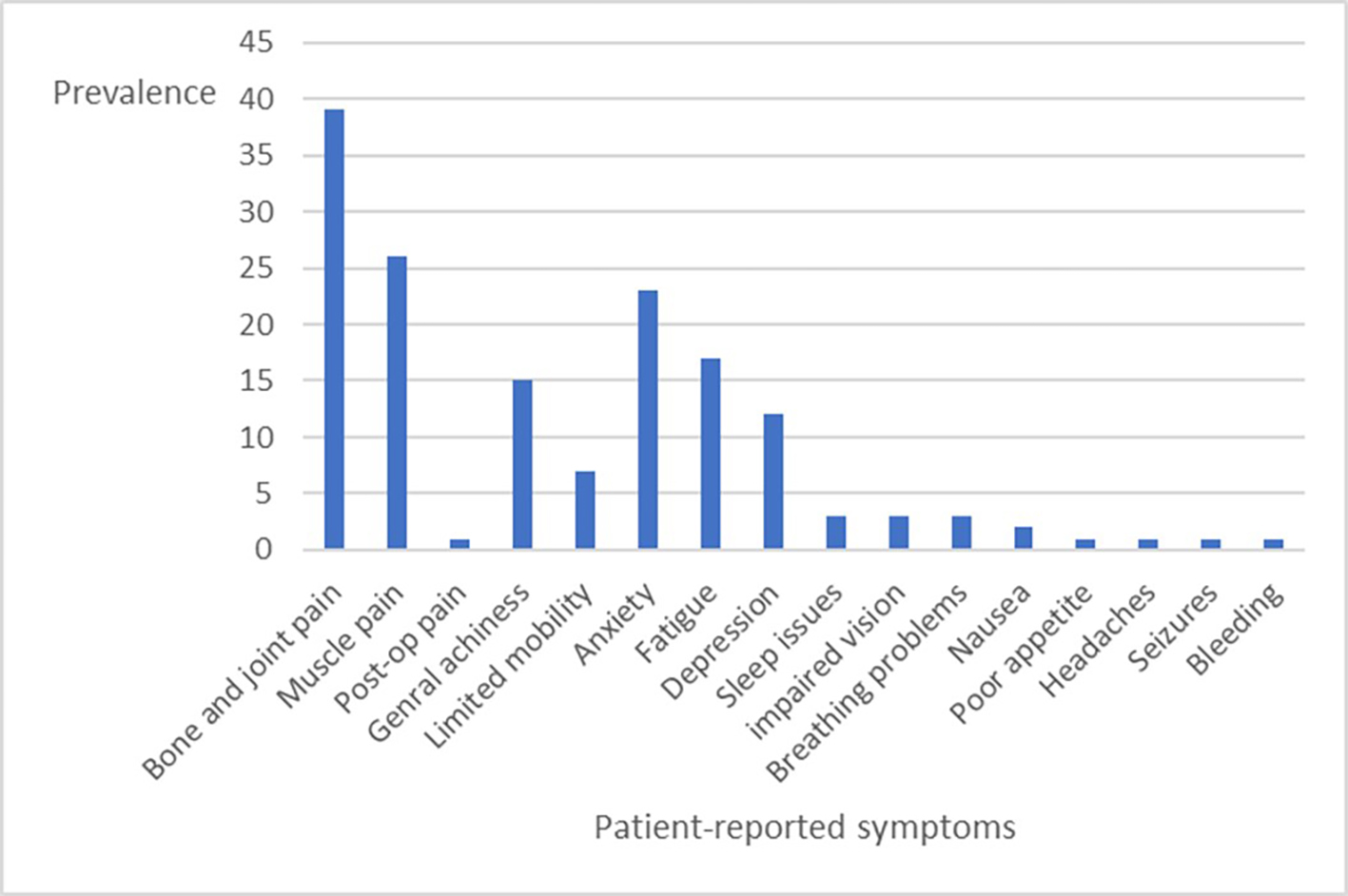
Sixty-two of 159 respondents (39%) reported current use of cannabis products primarily to ameliorate unrelieved symptoms they attributed to GD. The most common symptoms that were annotated included bone and joint pain, muscle pain, general achiness, anxiety, fatigue, depression, and limited mobility. Other symptoms were more sporadic: sleep issues, impaired vision, breathing problems, nausea, poor appetite, headaches, postoperative pain (shoulder), bleeding problems, and seizures [Figure 2]. 53% described their cannabis use as medicinal, 18% as recreational, and 29% as both medicinal and recreational. Only 26 of 51 respondents had spoken with their physicians about the use of cannabis products, which, during the most recent year, included cigarettes/joints, edible products, vaporizers, topical (i.e., cream, dermal patch), glass smoking apparatus (i.e., bowl, bong), oil, tea, wax, and pills/capsules. Cannabis formulations included Indica, Sativa, Hybrids, high CBD, THC-CBD gummies, low THC, equal THC/CBD, and Asteroids.
Figure 2. Prevalence of patient-reported symptoms associated with cannabis use among 51 of 62 GD patients who indicated use of such products. (11 patients did not answer the question).
There were no significant differences between the current age distribution of the patients who used cannabis and those who did not, nor in the age distribution at the time of GD diagnosis. The median age for first cannabis exposure was 20 years. 25% started cannabis use between the ages of 10 and 17 years. 25% first began using cannabis when they were more than 32 years old, including six aged 50 years or older who were all diagnosed with GD more than 20 years earlier. On the other hand, 16 patients began using cannabis before they were diagnosed with GD, including 10 in whom GD was diagnosed 20-40 years later. We cannot either assert or preclude the possibility that delayed diagnosis of GD contributed to the decision to use cannabis. The median age at which patients began using cannabis for what they reported to be medicinal reasons was 35 years, with all the patients aged 50 years or older included among the medicinal users.
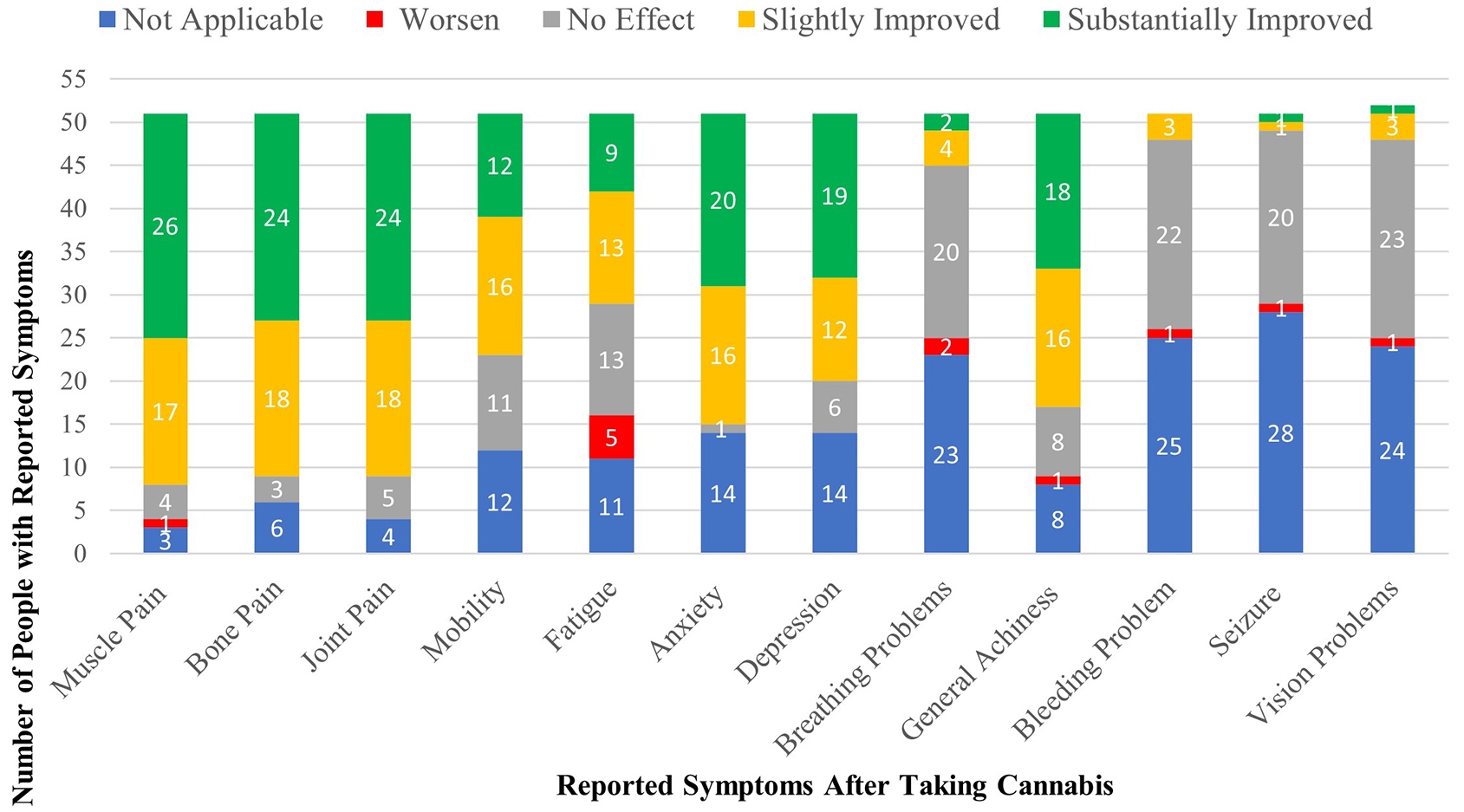
Table 1 and Figure 3 detail patient-reported assessments of the effect of cannabis on common symptoms of malaise. Regarding muscle, bone, and joint pain, nearly 50% reported substantial improvement with cannabis use and an additional ~34% reported slight improvement. A single patient with muscle pain felt worse. After subtracting the patients who had no pre-cannabis complaints of anxiety or depression (~28%), roughly equal numbers of patients reported significant or slight improvement. Regarding fatigue, 17.6% reported substantial improvement, 25.5% reported slight improvement, and 9.8% reported worsening. For bleeding and breathing problems, seizures, and vision problems, reports of improvement or worsening were negligible, as 84%-94% reported either “no effect” or “not applicable”.
Figure 3. Patient-reported assessments of the effect of cannabis on common symptoms of malaise. Regarding muscle, bone, and joint pain, nearly 50% reported substantial improvement with cannabis use (green bars) and an additional ~34% reported slight improvement (yellow bars). A single patient with muscle pain felt worse. After subtracting the patients who had no pre-cannabis complaints of anxiety or depression (~28%), roughly equal numbers of patients reported significant or slight improvement. Regarding fatigue, 17.6% reported substantial improvement, 25.5% reported slight improvement, and 9.8% reported worsening (red bars). For bleeding and breathing problems, seizures, and vision problems, reports of improvement or worsening were negligible, as 84%-94% reported either “no effect” or “not applicable”.
Patient-reported outcomes of cannabis use for the management of the different symptoms
| Symptoms affected by cannabis use | No effect # (%) | Not applicable # (%) | Slightly improved # (%) | Substantially improved # (%) | Improved at any level # (%) | Worse # (%) |
| Muscle pain | 4 (7.8) | 3 (5.8) | 17 (33.3) | 26 (51.0) | 43 (84.3) | 1 (2.0) |
| Bone pain | 3 (5.8) | 6 (11.8) | 18 (35.3) | 24 (47.1) | 42 (82.4) | |
| Joint pain | 5 (9.8) | 4 (7.8) | 18 (35.3) | 24 (47.1) | 42 (82.4) | 0 (0.0) |
| Mobility | 11 (21.6) | 12 (23.5) | 16 (31.4) | 12 (23.5) | 28 (54.9) | 0 (0.0) |
| Fatigue | 13 (25.5) | 11 (21.6) | 13 (25.5) | 9 (17.6) | 22 (43.1) | 5 (9.8) |
| Anxiety | 1 (2.0) | 14 (27.5) | 16 (31.4) | 20 (39.2) | 36 (70.6) | 0 (0.0) |
| Depression | 6 (11.8) | 14 (27.5) | 12 (23.5) | 19 (37.3) | 31 (60.8) | 0 (0.0) |
| Breathing problems | 20 (39.2) | 23 (45.1) | 4 (7.8) | 2 (3.9) | 6 (11.8) | 2 (4.0) |
| General achiness | 8 (15.7) | 8 (15.7) | 16 (31.4) | 18 (35.3) | 34 (66.7) | 1 (2.0) |
| Bleeding problem | 22 (43.1) | 25 (49.0) | 3 (5.9) | 0 (0.0) | 3 (5.9) | 1 (2.0) |
| Seizure | 20 (39.2) | 28 (54.9) | 1 (2.0) | 1 (2.0) | 2 (3.9) | 1 (2.0) |
| Vision problems | 23 (45.1) | 24 (47.1) | 3 (5.9) | 1 (2.0) | 4 (7.8) | 1 (2.0) |
Critical evaluations of these results are impossible in the absence of an adequate, placebo-controlled group. However, we can compare the cannabis and non-cannabis users according to the status of GD-associated symptoms at the time of the questionnaire submission [Table 2]. Among patients reporting no or only mild symptoms, there was no difference in the number of cannabis users compared to non-users. However, among the smaller number of GD patients who reported moderate/extreme symptomatology, there was a disproportionately larger number of cannabis users than those who have never used cannabis products
Symptomatic differences between patients using cannabis products and those not using such products (expressed as percentages of patients not using cannabis (N = 98) and those using cannabis (N = 62)
| Current symptoms | Not sure | Not at all/mild | Moderate/extreme | |||
| Using cannabis | No | Yes | No | Yes | No | Yes |
| N = 98 | N = 62 | N = 98 | N = 62 | N = 98 | N = 62 | |
| Enlarged spleen | 1.1 | 1.8 | 88.8* | 84.0# | 10.1# | 14.3* |
| Thrombocytopenia | 1.1 | 1.8 | 91.0* | 85.7# | 7.9# | 12.5* |
| Bleeding problem | 1.1 | 0.0 | 95.5* | 92.8# | 3.3# | 7.2* |
| Frequent fatigue | 0.0 | 0.0 | 64.1* | 50.0# | 36.0# | 50.0* |
| Breathing problem | 0.0 | 1.1 | 94.4* | 87.5# | 5.6# | 10.8* |
| (Bone) osteoporosis | 1.1 | 3.6 | 75.3* | 62.9# | 23.6# | 33.5* |
| Bone or joint pain | 0.0 | 0.0 | 62.9* | 63.1# | 37.1# | 36.9* |
| Bone or joint damage | 6.7 | 5.4 | 69.6* | 62.5# | 23.7# | 32.1* |
| Prone to bone fracture | 7.9 | 1.3 | 85.4* | 82.2# | 6.7# | 16.5* |
| Bone or joint surgery | 1.1 | 0.0 | 87.6* | 91.1# | 11.3# | 8.9* |
| Poor peripheral vision | 3.4 | 3.5 | 94.4* | 94.7# | 2.2# | 1.8* |
| Memory problem | 2.2 | 0.0 | 93.3* | 84.0# | 4.5# | 16.0* |
| Coordination problem | 3.4 | 8.9 | 91.0* | 87.5# | 5.6# | 3.6* |
| Seizures | 3.4 | 0.0 | 96.7* | 98.2# | 0.0# | 1.8* |
| χ2 (RT P-value): 0.702 | χ2 (RT P-value): 0.001 | |||||
This survey was not designed to assess cannabis efficacy or safety in patients with GD1. However, we wished to explore whether we could detect any differences in patient-reported outcomes based on a simplistic assessment of total cannabis dose, disregarding any considerations of differences in cannabis product, composition, and route of administration. For this purpose, we calculated an estimated number of lifelong cannabis days for each cannabis user. The range was very large: fewer than 100 days to nearly 100,000 days, necessitating the division of the 62 cannabis users into four subgroups (underpowered for meaningful statistical analysis).
The percentages of individuals stratified by lifelong cannabis use who reported symptoms of moderate or extreme severity are shown in Supplementary Figure 1A. The percentages of those either asymptomatic for a specific clinical manifestation or only mildly affected are depicted in Supplementary Figure 1B and
DISCUSSION
The primary objective of this exploratory study was to confirm our suspicion that adult patients with GD1, particularly those with moderate to severe persistent symptoms, have historically used cannabis products to try to alleviate those symptoms. Secondarily, we wished to probe to what extent non-validated patient-reported outcomes (symptom relief and adverse side effects) were related to the frequency and duration of cannabis use. We believe that we have achieved both objectives with the survey and can now make a credible case for future, more rigorous research studies with far fewer limitations than those associated with this study.
Gallup’s 2021 report, based on a large general US population, showed that 45% of all American adults have tried marijuana at some time in their lives, solo or socially, for various reasons including mood elevation, relaxation, and enjoyment (recreationally), or to alleviate discomforting physical or psychological symptoms (medicinally)[43]. In 2021-2022, recreational use of marijuana was illegal in all but 11 US states[44]. Therefore, the Gallup results may be an underestimate due to reluctance to report criminal activity even under a shield of anonymity. However, despite less than compelling evidence for efficacy and safety, multiple studies indicate that a substantial plurality of patients with chronic autoimmune, inflammatory and degenerative diseases report the use of medical cannabis, including newer and increasingly popular products that are refined and purified beyond traditional “pot” and “weed”[31-36,45-52]. Often, these products are obtained from dispensaries without prior or post-hoc knowledge of the patient’s physician(s)[53]. From 2021-2023, among 175,734 patients screened during annual wellness visits at a large urban university primary care clinic, 17% reported cannabis use (edibles, smoking and vaping) and 5.9% had moderate to high risk for cannabis use disorder. 76% reported using cannabis for the management of symptoms including pain, stress, and sleep disorders[54].
Given this background, it hardly seems surprising that 39% of our survey respondents reported using cannabis even after they had been started on treatment for GD1, with only half having discussed the use of cannabis with their physicians.
Despite the proven efficacy of ERT/SRT for GD1, some patients continue to have unmet needs, particularly in terms of health-related quality of life symptoms such as chronic pain, persistent fatigue, anxiety, and depressed mood[55]. In 2009, Balwani et al. surveyed 237 GD1 patients at a single academic GD center regarding the use of complementary and alternative medicine (e.g., glucosamine, chondroitin, antioxidants omega-3 fatty acids, Echinacea and methylsulfonylmethane)[56]. Among 83 adult GD1 respondents, 28 (34%) used one or more of these therapies to alleviate residual symptoms, albeit rarely with objective beneficial effects[56]. However, these patients were not asked about cannabis/marijuana. Our study is the first to collect information about the use of cannabis products by patients with GD1 (Pub Med verified 24 May 2024).
From the outset, recognizing our sparse resources including local IRB regulations restricting even indirect access to GD patients followed at academic centers, we knew that this study would have many limitations and we adjusted our outcome expectations accordingly. However, we submit that our cohort of 159 respondents is reasonably representative of the population of adult US patients with GD1 who are receiving ERT/SRT (“guesstimate” ~4,000; NJW personal communication) and see Supplementary Table 1. The percentage of respondents who report Ashkenazi Jewish descent (50%) is in line with other US GD study cohorts[6,57-60]. Despite the large representation of Ashkenazi Jews, the majority of the 159 respondents reported having moderate to extreme splenomegaly, thrombocytopenia, and bone manifestations before starting treatment. This degree of pre-treatment disease severity resembles the mean DS3 severity score of 5.6 ± 2.3 reported in a cohort of 133 adult patients with GD1 enrolled at five geographically dispersed North American GD treatment centers (3.0-5.9: moderate; ≥ 6.0: severe)[59].
To limitations cited earlier in the manuscript text, we reiterate or add:
• Lack of quantitative definitions for symptom severity or validated QOL tools.
• Lack of objective clinical data to substantiate or refute subjective patient-reported outcomes.
• Information concerning pre-treatment manifestations was likely based on memory recall alone and may therefore be unintentionally inaccurate.
• There are only two assessment points that, for many patients, span years of their lives and are not necessarily representative of their overall health status. We did not collect information on whether the reported pain or other symptoms were constant or intermittent.
• Lack of data about concurrent illnesses or medications.
• Lack of validation of the questionnaire (other than a limited degree of face validation and review by one GD1 patient).
• Possible reporting bias based on personal beliefs about cannabis legalization.
Advocates may be more motivated to report usage. Opponents may be motivated to make a statement by reporting abstinence.
• Individuals who tried cannabis with no response or with adverse effects may be less likely to have been study participants.
• Small sample sizes in stratified cannabis user cohorts.
• Lack of specific questions about possible cannabis use disorder.
• The wide variety of Cannabis formulations used by patients does not allow for a reliable analysis of whether any are superior in effectiveness or in reducing the known adverse effects induced by the drug.
Why should the results of this admittedly preliminary survey study be important for physicians and allied health care professionals who care for patients with GD1, and especially those who work at specialized GD centers? Surveys showing that, with the exception of elderly respondents (> 65 years old), 88% of US adults favor legalization of marijuana for either medical or recreational use[61], suggest that many are convinced that self-prescribed regulated and even unregulated cannabis products are safe and generally free of deleterious side effects[62]. However, cannabis use disorder and possible risks for longer-term mental health consequences are legitimate concerns even in nominally healthy individuals and particularly in adolescents[63]. In patients with rare diseases and unique pathophysiology such as GD, there is currently no knowledge of whether cannabis use might lead to disease-specific effects. Some investigators caution against cannabis use because of its possible link to liver toxicity and drug-drug interactions - subjects relevant to patients with GD[64]. Decreased bone mineral density (BMD) with increased fracture risk is common in patients with GD1, but the effects of chronic cannabis use on BMD in untreated and ERT/SRT-treated patients are unknown. The literature on cannabis and bone mineral density suggests that effects may vary depending on the type of cannabis product and route of administration. Compared to 114 cigarette smokers (a known risk factor for osteopenia/osteoporosis[65]), 144 heavy cannabis smokers had low BMD, increased bone turnover, and increased fracture risk[66]. However, other pre-clinical studies suggest that purified cannabis derivatives, such as cannabidiol (CBD), may improve BMD[67]. Episodic osteonecrosis occurs in untreated and treated patients with GD1. In a solitary study of patients with sickle cell anemia, osteonecrosis and bone crises were more common in 69 cannabis users than in 155 non-users[68]. However, it was uncertain as to whether the severity of bone crises and subsequent osteonecrosis prompted cannabis use or vice versa.
Compared to individuals with no GBA1 variants or other mutations associated with Parkinson’s disease (PD) risk, patients with GD1 (and, to a lesser extent, GBA1 carriers) have an increased probability of presenting with overt PD and its variants such as Lewy body dementia beginning around age 50 years and rising to approximately 8% by age 80[4]. Cannabis products have been used empirically and in several controlled clinical trials to treat both motor and non-motor manifestations of PD with comprehensive review articles reporting equivocal and even contradictory conclusions[69-72]. Involvement of endocannabinoid receptors and signaling in PD are thought to be likely, but the mechanisms and consequences are far from clear[73]. Thus, there is no evidence that the use of cannabis products abets, prevents, or is neutral regarding GBA1-associated PD. The memory loss, impaired attention, and dyscoordination reported by our study respondents with the greatest use of cannabis is most likely a direct effect of tetrahydrocannabinol exposure and, based on current knowledge, need not be interpreted as increasing risk for PD or other neurodegenerative disorder[74].
Studies from the ICGG Registry and other sources indicate that patients with GD1 have an increased risk for developing monoclonal gammopathy of uncertain significance (MGUS), myeloma, B cell lymphoma, myelodysplasia, acute leukemia, hepatocellular carcinoma, and renal cell carcinoma[6]. There is little evidence implicating cannabis use in the emergence of malignancies. In fact, past cannabis use was associated with a decreased risk for urothelial cancers in women, including renal cell carcinoma[75]. After correcting for risks associated with smoking tobacco, there is little evidence for an increased risk of non-small cell lung cancer among habitual or long-term cannabis smokers[76].
CONCLUSION
This is the first study to document cannabis use by patients with type 1 GD and to examine the possibility that adjunctive cannabis use may improve residual symptoms that are not resolved solely with ERT/SRT. Within the limitations of the study, we find that 39% of our survey respondents used cannabis products, but that frequency and continuity of use were highly variable, making it difficult to assess symptomatic efficacy. In general, cannabis users reported improvements in pain (bone, joint and muscle), anxiety, and depression. However, impaired mobility, breathing problems, and fatigue had relatively little improvement. Patients who used cannabis most frequently and consistently were more likely to report adverse effects known to be caused by cannabis, including memory loss, impaired attention, and dyscoordination.
In theory, a definitive determination that cannabis can safely benefit chronically symptomatic GD1 patients should require adequately powered RCTs using standardized products and placebo controls. Realistically, with the uncontrolled proliferation of cannabis providers and the momentum favoring the legalization of cannabis for recreational use, the prospect for such studies is dim. As an alternative, for diseases such as GD that already have strong existent anonymized disease registries, additional data collection and observational analysis about cannabis use and other adjunctive treatments should be feasible. Although some patients may be reluctant to talk openly with their doctors about cannabis use, they should be routinely queried about such use by primary care physicians and GD specialists who, in turn, need to be sufficiently educated to provide informed guidance on safety, dosage, and potential interactions with other medications the patient is using.
DECLARATIONS
Authors’ contributions
Conception and design of the project, recruitment letter, data analysis, review of relevant literature, manuscript revision: Berman Y
Conception and design of the project, review of relevant literature, manuscript revision: Ely R
Data and statistical analyses, preparation of tables and figures, manuscript revision: Scher YY
Conception and design of the project, IRB submission, recruitment letter, data and statistical analysis, review of relevant literature, writing of the original draft, manuscript revision: Weinreb NJ
Availability of data and materials
Copies of the Study Protocol and the Survey Questionnaire are provided in Supplementary Material. The data supporting these findings are stored in Dr Neal Weinreb’s office in Boca Raton, FL, USA and will be available upon reasonable request with the consent of all the authors.
Financial support and sponsorship
University Research Foundation for Lysosomal storage Diseases Inc. paid the fee for the waiver approval application to WRB IRB, Seattle, WA, USA. Minor administrative expenses were paid personally by the study investigators. The study was not commissioned by any commercial company/organization nor sponsored by any industrial entity.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The research qualified for a waiver of authorization for use and disclosure of protected health information after review by WCG IRB (23 DEC 2020). Completion of the survey was indicative of voluntary consent, as explained in the disclosure document and survey introduction.
Consent for publication
To preserve anonymity, the email addresses of participants (needed for gift card distribution) were not retained.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
1. Castillon G, Chang SC, Moride Y. Global incidence and prevalence of gaucher disease: a targeted literature review. J Clin Med. 2022;12:85.
2. Nalysnyk L, Rotella P, Simeone JC, Hamed A, Weinreb N. Gaucher disease epidemiology and natural history: a comprehensive review of the literature. Hematology. 2017;22:65-73.
3. Daykin EC, Ryan E, Sidransky E. Diagnosing neuronopathic Gaucher disease: new considerations and challenges in assigning Gaucher phenotypes. Mol Genet Metab. 2021;132:49-58.
4. Horowitz M, Braunstein H, Zimran A, Revel-Vilk S, Goker-Alpan O. Lysosomal functions and dysfunctions: molecular and cellular mechanisms underlying Gaucher disease and its association with Parkinson disease. Adv Drug Deliv Rev. 2022;187:114402.
5. Hughes D, Mikosch P, Belmatoug N, et al. Gaucher disease in bone: from pathophysiology to practice. J Bone Miner Res. 2019;34:996-1013.
6. Rosenbloom BE, Cappellini MD, Weinreb NJ, et al. Cancer risk and gammopathies in 2123 adults with Gaucher disease type 1 in the International Gaucher Group Gaucher Registry. Am J Hematol. 2022;97:1337-47.
7. Roh J, Subramanian S, Weinreb NJ, Kartha RV. Gaucher disease - more than just a rare lipid storage disease. J Mol Med. 2022;100:499-518.
8. Weinreb NJ, Barbouth DS, Lee RE. Causes of death in 184 patients with type 1 Gaucher disease from the United States who were never treated with enzyme replacement therapy. Blood Cells Mol Dis. 2018;68:211-7.
9. Revel-Vilk S, Szer J, Mehta A, Zimran A. How we manage Gaucher disease in the era of choices. Br J Haematol. 2018;182:467-80.
10. Weinreb NJ, Camelo JS Jr, Charrow J, McClain MR, Mistry P, Belmatoug N. International Collaborative Gaucher Group (ICGG) Gaucher Registry (NCT00358943) investigators. Gaucher disease type 1 patients from the ICGG Gaucher registry sustain initial clinical improvements during twenty years of imiglucerase treatment. Mol Genet Metab. 2021;132:100-11.
11. Andrade-Campos MM, de Frutos LL, Cebolla JJ, et al. Identification of risk features for complication in Gaucher's disease patients: a machine learning analysis of the Spanish registry of Gaucher disease. Orphanet J Rare Dis. 2020;15:256.
12. Goker-Alpan O. Therapeutic approaches to bone pathology in Gaucher disease: past, present and future. Mol Genet Metab. 2011;104:438-47.
13. Grabowski GA, Antommaria AHM, Kolodny EH, Mistry PK. Gaucher disease: basic and translational science needs for more complete therapy and management. Mol Genet Metab. 2021;132:59-75.
14. Sam R, Ryan E, Daykin E, Sidransky E. Current and emerging pharmacotherapy for Gaucher disease in pediatric populations. Expert Opin Pharmacother. 2021;22:1489-503.
15. Els C, Jackson TD, Kunyk D, et al. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;10:CD012509.
16. Noori A, Sadeghirad B, Wang L, et al. Comparative benefits and harms of individual opioids for chronic non-cancer pain: a systematic review and network meta-analysis of randomised trials. Br J Anaesth. 2022;129:394-406.
17. Almogi-Hazan O, Or R. Cannabis, the endocannabinoid system and immunity-the journey from the bedside to the bench and back. Int J Mol Sci. 2020;21:4448.
18. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9-29.
19. Crocq MA. History of cannabis and the endocannabinoid system. Historia del cannabis y del sistema de endocannabinoides. Histoire du cannabis et du système endocannabinoϊde. Dialogues Clin Neurosci. 2020;22:223-8.
20. Lowin T, Schneider M, Pongratz G. Joints for joints: cannabinoids in the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2019;31:271-8.
21. Weiss L, Zeira M, Reich S, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143-51.
22. Gallily R, Yekhtin Z, Hanuš LO. The anti-inflammatory properties of terpenoids from cannabis. Cannabis Cannabinoid Res. 2018;3:282-90.
23. Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants. 2019;9:21.
24. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453-62.
25. Cherukury HM, Argueta DA, Garcia N, et al. Cannabidiol attenuates hyperalgesia in a mouse model of sickle cell disease. Blood. 2023;141:203-8.
26. Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101-22.
27. Xin Y, Tang A, Pan S, Zhang J. Components of the endocannabinoid system and effects of cannabinoids against bone diseases: a mini-review. Front Pharmacol. 2021;12:793750.
28. Raphael-Mizrahi B, Gabet Y. The cannabinoids effect on bone formation and bone healing. Curr Osteoporos Rep. 2020;18:433-8.
29. Lu HC, Mackie K. Review of the endocannabinoid system. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:607-15.
30. Florida Cannabis Information. Is CBD oil legal in Florida? Available from: FloridaStateCannabis.org [Last accessed on 22 Aug 2024].
31. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812-9.
32. Collin C, Davies P, Mutiboko IK, Ratcliffe S. Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14:290-6.
33. Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182:E694-701.
34. Naftali T, Bar-Lev Schleider L, Scklerovsky Benjaminov F, Konikoff FM, Matalon ST, Ringel Y. Cannabis is associated with clinical but not endoscopic remission in ulcerative colitis: a randomized controlled trial. PLoS One. 2021;16:e0246871.
35. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225-31.
36. Longo R, Oudshoorn A, Befus D. Cannabis for chronic pain: a rapid systematic review of randomized control trials. Pain Manag Nurs. 2021;22:141-9.
37. Fisher E, Moore RA, Fogarty AE, et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain. 2021;162:S45-66.
38. Arkell TR, Downey LA, Hayley AC, Roth S. Assessment of medical cannabis and health-related quality of life. JAMA Netw Open. 2023;6:e2312522.
39. Abuhasira R, Schleider LBL, Mechoulam R, Novack V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med. 2018;49:44-50.
40. Costa B. On the pharmacological properties of Δ9-tetrahydrocannabinol (THC). Chem Biodivers. 2007;4:1664-77.
41. Holden SK, Domen CH, Sillau S, Liu Y, Leehey MA. Higher risk, higher reward? Self-reported effects of real-world cannabis use in parkinson's disease. Mov Disord Clin Pract. 2022;9:340-50.
42. Chesney E, Oliver D, Green A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45:1799-806.
43. Jones JM. Nearly half of U.S. adults have tried marijuana. Available from: https://news.gallup.com/poll/353645/nearly-half-adults-tried-marijuana.aspx [Last accessed on 22 Aug 2024].
44. ProCon.org. State-by-state recreational marijuana laws. Available from: https://marijuana.procon.org/legal-recreational-marijuana-states-and-dc/ [Last accessed on 22 Aug 2024].
45. Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891-6.
46. Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: a population based analysis. Drug Alcohol Depend. 2015;156:84-9.
47. Buckley MC, Kumar A, Swaminath A. Inflammatory bowel disease and cannabis: a practical approach for clinicians. Adv Ther. 2021;38:4152-61.
48. Wipfler K, Simon T, Katz P, et al. Cannabis use among patients in a large US rheumatic disease registry. In: 2019 ACR/ARP annual meeting. Available from: https://acrabstracts.org/abstract/cannabis-use-among-patients-in-a-large-us-rheumatic-disease-registry/ [Last accessed on 22 Aug 2024].
49. Nielsen SW, Ruhlmann CH, Eckhoff L, Brønnum D, Herrstedt J, Dalton SO. Cannabis use among Danish patients with cancer: a cross-sectional survey of sociodemographic traits, quality of life, and patient experiences. Support Care Cancer. 2022;30:1181-90.
50. Cousins MM, Mayo C, Devasia T, et al. Cannabis use in patients seen in an academic radiation oncology department. Pract Radiat Oncol. 2023;13:112-21.
51. Cousins MM, Jannausch ML, Coughlin LN, Jagsi R, Ilgen MA. Prevalence of cannabis use among individuals with a history of cancer in the United States. Cancer. 2021;127:3437-44.
52. Johal H, Devji T, Chang Y, Simone J, Vannabouathong C, Bhandari M. Cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120906461.
53. Powell A. Medical school’s Kevin hill talks about fearmongering and rosy myths, safe use and addiction. Available from: https://news.harvard.edu/gazette/story/2020/02/professor-explores-marijuanas-safe-use-and-addiction/ [Last accessed on 22 Aug 2024].
54. Gelberg L, Beck D, Koerber J, et al. Cannabis use reported by patients receiving primary care in a large health system. JAMA Netw Open. 2024;7:e2414809.
55. Elstein D, Belmatoug N, Deegan P, et al. Development and validation of Gaucher disease type 1 (GD1)-specific patient-reported outcome measures (PROMs) for clinical monitoring and for clinical trials. Orphanet J Rare Dis. 2022;17:9.
56. Balwani M, Fuerstman L, Desnick RJ, Buckley B, McGovern MM. Use of complementary and alternative medicine by patients with lysosomal storage diseases. Genet Med. 2009;11:722-7.
57. Mistry PK, Balwani M, Charrow J, et al. Real-world effectiveness of eliglustat in treatment-naïve and switch patients enrolled in the international collaborative Gaucher group Gaucher registry. Am J Hematol. 2020;95:1038-46.
58. Zimran A, Belmatoug N, Bembi B, et al. GOS study group. Demographics and patient characteristics of 1209 patients with Gaucher disease: descriptive analysis from the Gaucher outcome survey (GOS). Am J Hematol. 2018;93:205-12.
59. Weinreb NJ, Finegold DN, Feingold E, et al. Evaluation of disease burden and response to treatment in adults with type 1 Gaucher disease using a validated disease severity scoring system (DS3). Orphanet J Rare Dis. 2015;10:64.
60. Mistry PK, Batista JL, Andersson HC, et al. Transformation in pretreatment manifestations of Gaucher disease type 1 during two decades of alglucerase/imiglucerase enzyme replacement therapy in the international collaborative Gaucher group (ICGG) Gaucher registry. Am J Hematol. 2017;92:929-39.
61. Most Americans favor legalizing marijuana for medical, recreational use. 2024. Available from: www.pewresearch.org/wp-content/uploads/sites/20/2024/03/PP_2024.3.26_marijuana_REPORT.pdf [Last accessed on 22 Aug 2024].
62. Luc MH, Tsang SW, Thrul J, Kennedy RD, Moran MB. Content analysis of online product descriptions from cannabis retailers in six US states. Int J Drug Policy. 2020;75:102593.
63. Walker M, Carpino M, Lightfoot D, et al. The effect of recreational cannabis legalization and commercialization on substance use, mental health, and injury: a systematic review. Public Health. 2023;221:87-96.
64. Sholler DJ, Schoene L, Spindle TR. Therapeutic efficacy of cannabidiol (CBD): a review of the evidence from clinical trials and human laboratory studies. Curr Addict Rep. 2020;7:405-12.
65. Joehanes R, Just AC, Marioni RE, et al. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet. 2016;9:436-47.
66. Sophocleous A, Robertson R, Ferreira NB, McKenzie J, Fraser WD, Ralston SH. Heavy cannabis use is associated with low bone mineral density and an increased risk of fractures. Am J Med. 2017;130:214-21.
67. Ihejirika-Lomedico R, Patel K, Buchalter DB, et al. Non-psychoactive cannabidiol prevents osteoporosis in an animal model and increases cell viability, proliferation, and osteogenic gene expression in human skeletal stem and progenitor cells. Calcif Tissue Int. 2023;112:716-26.
68. Miodownik H, Curtis SA, Olivia Ogu U, et al. Frequent health care utilisation and avascular necrosis are associated with cannabis use in adults with sickle cell disease. Br J Haematol. 2022;196:e41-4.
69. Urbi B, Corbett J, Hughes I, et al. Effects of cannabis in parkinson's disease: a systematic review and meta-analysis. J Parkinsons Dis. 2022;12:495-508.
70. Figura M, Koziorowski D, Sławek J. Cannabis in parkinson’s disease - the patient’s perspective versus clinical trials: a systematic literature review. Neurol Neurochir Pol. 2022;56:21-7.
71. Varshney K, Patel A, Ansari S, Shet P, Panag SS. Cannabinoids in treating parkinson’s disease symptoms: a systematic review of clinical studies. Cannabis Cannabinoid Res. 2023;8:716-30.
72. Ferreira-Junior NC, Campos AC, Guimarães FS, et al. Biological bases for a possible effect of cannabidiol in Parkinson’s disease. Braz J Psychiatry. 2020;42:218-24.
73. Fernández-Moncada I, Eraso-Pichot A, Dalla Tor T, Fortunato-Marsol B, Marsicano G. An enquiry to the role of CB1 receptors in neurodegeneration. Neurobiol Dis. 2023;184:106235.
75. Huang J, Huang D, Ruan X, et al. Association between cannabis use with urological cancers: a population-based cohort study and a mendelian randomization study in the UK biobank. Cancer Med. 2023;12:3468-76.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].