fig1
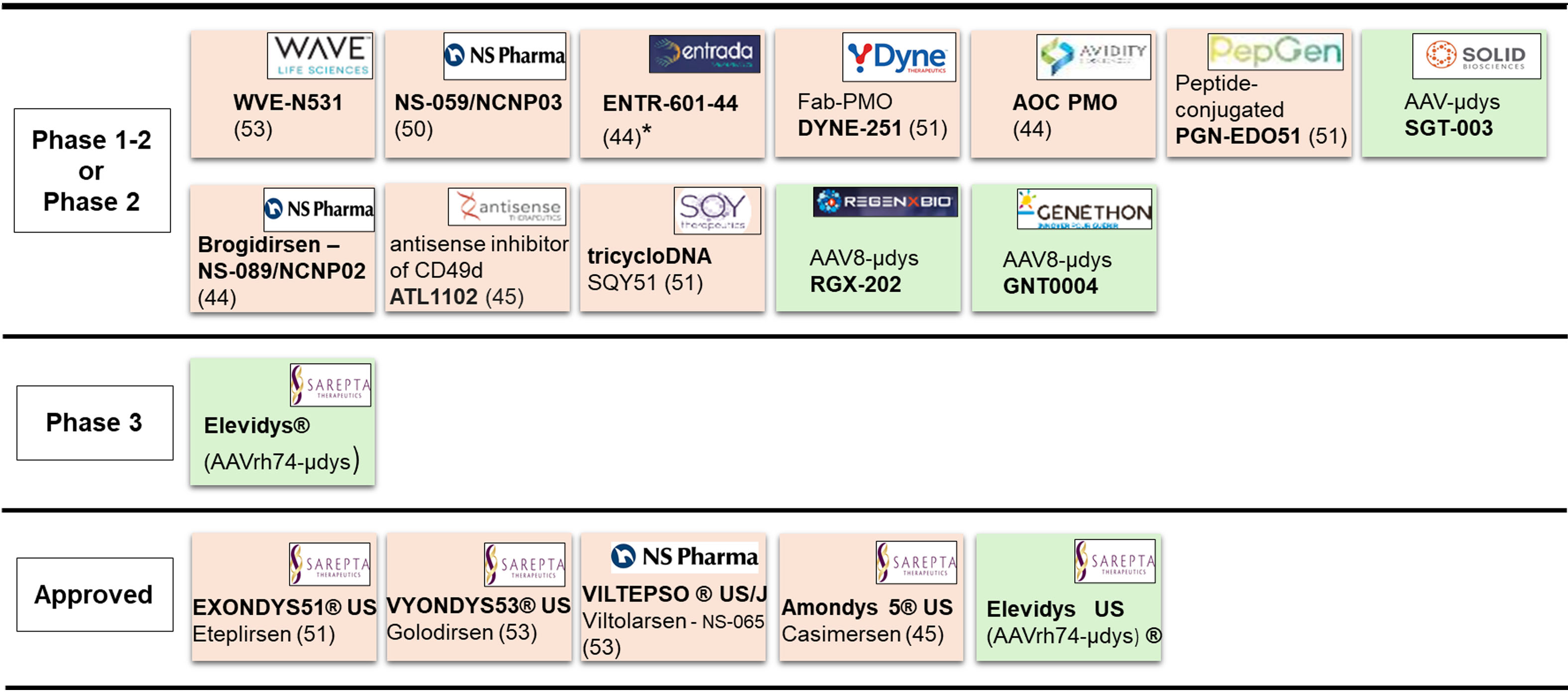
Figure 1. Current pipeline of gene-based therapies. Positioning of the antisense (beige) or gene-replacement (green) therapies, according to the stage of development (left column). Bold: drug name including commercial name (®); US: approved in USA; J: approved in Japan; Logos: name of the sponsor; Number in parentheses: targeted exon; *: clinical hold by FDA; µdys: microdystrophin; Fab-PMO: antibody-conjugated Phosphorodiamidate Morpholino Oligomers. Two other studies carried out in China (NCT06114056 relative to an AAV-microdystrophin, and NCT06594094 relative to a CRISPR-gene editing approach) are not indicated here. PMO: Phosphorodiamidate morpholino oligomer; AAV: Adeno-associated virus; FDA: Food and drug administration.









