The underdiagnosed kidney burden of Fabry disease in females
Abstract
Aim: Progressive kidney deterioration can occur in females with Fabry disease. Despite this, many females go undiagnosed or are labeled “carriers”. Our study aimed to review biopsy findings of adult female Fabry patients with creatinine values within the “normal reference ranges” and minimal proteinuria.
Methods: This study was a single-center retrospective analysis of female patients presenting to the University of Alabama at Birmingham. Kidney biopsies and clinical characteristics were reviewed. Kidney biopsies were scored using the Fogo scoring system.
Results: All of the 13 participants had classic mutations (11 nonsense, 2 missense). The mean age was 31.7 years. The median serum creatinine was 0.7 mg/dL (0.5-1.2), and estimated glomerular filtration rate (eGFR) values calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation ranged from 56 to 130 ml/min/1.73m2. The serologic biomarkers were not elevated in any of the patients - plasma globotriaosylsphingosine (Lyso GL-3) was < 5.1 ng/mL and globotriaosylceramide (GL-3) was < 3 µg/mL. The average score for the biopsies per the International Study Group of Fabry Nephropathy (ISGFN) was 2.43. Thirty-eight percent of patients had greater than 50% foot process effacement despite normal creatinine and minimal proteinuria.
Conclusion: Females with Fabry disease, despite having normal reference range creatinine, minimal proteinuria, and low plasma Lyso GL-3, had significant histologic evidence of the disease. When possible, histologic assessment should be performed in females to assess tissue involvement. Longitudinal follow-up is needed to determine if histologic findings on presentation predict long-term outcomes.
Keywords
INTRODUCTION
Fabry disease (FD) is the most prevalent lysosomal storage disorder. It is an X-linked inborn error of the glycosphingolipid metabolic pathway due to the deficiency of α-galactosidase A (GLA) that results in lysosomal accumulation of globotriaosylceramide (GL-3) and its derivative globotriaosylsphingosine (Lyso GL-3) in various cells, leading to clinical manifestations. Females with FD are heterozygous for the Fabry gene mutation[1,2].
Historically, females were considered asymptomatic “carriers” of the defective GLA gene and invulnerable to the clinical manifestations of FD. However, it is now known that heterozygous females have a variable course that ranges from asymptomatic disease to a severe phenotype resembling that in males[3-6]. These observed differences in the phenotypic variation may be due to skewed X-chromosome random inactivation, resulting in a higher percentage of cells with active X chromosomes expressing the mutation. Consequently, the affected cells have less or no GLA-enzyme activity, affecting vital organ functions (kidney, heart, and brain)[7].
When renal involvement occurs, glycosphingolipids accumulate in the glomeruli, particularly the podocytes, leading to proteinuria. If not detected and treated early, this can cause a progressive decline in kidney function[8]. Female manifestations of kidney disease often go under-recognized for multiple reasons. The serum creatinine measurement often underestimates renal dysfunction in women due to reduced muscle mass[9]. For any given estimated glomerular filtration rate (eGFR) value, creatinine levels are usually lower in females versus male counterparts despite having similar normal creatinine reference ranges. Females can have a significant decline in eGFR despite having creatinine values in the normal reference range. In addition, during the collection of the data, most hospitals reported the eGFR using the Modification of Diet in Renal Disease (MDRD) equation, a formula whose accuracy declines at higher GFR levels; thus, only values less than an eGFR of 60 mL/min/1.73m2 are reported[10]. Biomarkers, specifically GL-3 and Lyso GL-3, are detected in the plasma and urine of patients with FD. However, GL-3 and
Kidney biopsies have proven reliable in diagnosing and evaluating FD's histologic involvement. However, due to their invasive nature, biopsies are not typically the first choice as a diagnostic tool and are usually reserved for patients with elevated creatinine or those with more than 500 mg of proteinuria per day[13]. As many as 40% of heterozygous females have kidney dysfunction, but there is a paucity of data on the early pathological changes and kidney biopsy findings in females[14].
Renal biopsies were performed based on clinical suspicion of Fabry nephropathy, even in the absence of proteinuria. We have analyzed and summarized the kidney biopsy findings in female patients with FD whose creatinine at the time of biopsy was in the normal range and who had minimal proteinuria of < 500 mg/day by urine protein-creatinine ratio (UPCR).
METHODS
Patients and patient characteristics
Females with FD diagnosis who underwent kidney biopsies at the University of Alabama at Birmingham between 2000 and 2020 were retrieved.
Patients with elevated serum creatinine, proteinuria > 500 mg/day by UPCR, and those with incomplete serological data or who did not undergo genetic testing were excluded from the analysis. Patients on medications known to cause intracellular inclusion bodies, such as amiodarone and chloroquine, were excluded.
The presence and severity of FD’s symptoms, such as acroparasthesia, hypohidrosis/hyperhydrosis, gastrointestinal symptoms, cornea verticillate, and angiokeratoma, were collected. Information on the presence of left ventricular hypertrophy by two-dimensional (2D) echocardiography, history of hypertension, and anti-hypertensive medication use was also collected. Patients were not on enzyme replacement therapy (ERT) or any other FD-specific therapy at the time of biopsy.
The study protocol was approved by the Institution Review Board at the University of Alabama in Birmingham.
Kidney biopsy
Renal core biopsies from each patient, received fresh in a transport media (RPMI with L-glutamine), were triaged for light microscopy (LM), Immunofluorescence (IF), and electron microscopy (EM). For LM, a portion of cores were fixed in 10% buffered formalin processed in the usual manner and paraffin-embedded; 2-3 μm sections were stained with hematoxylin-eosin, Periodic acid Schiff hematoxylin (PASH), trichrome, and Jones silver. For IF, 4-6 mm cores were placed in OCT (Optimal Cutting Temperature compound) and quickly frozen at -20 °C; 2-3 μm sections were stained using antisera for IgG, IgA, IgM, C3, C1q, and lambda light chain (MP Biomedicals, LLC. Solon, Ohio) and kappa light chain [Agilent Technologies Singapore (International) Pte Ltd]. For EM, 1-2 mm pieces were initially fixed in 10% Carson's neutral buffered formalin, post-fixed in 1% osmium tetroxide, and embedded in epoxy resin. Ultrathin sections
Kidney biopsy scoring and interpretation
Standard evaluation that includes light microscopy, immunofluorescence, and electron microscopy was done for kidney biopsy samples. A validated scoring system for renal pathology in FD developed by Fogo and members of the International Study Group of Fabry Nephropathy (ISGFN) was used[13]. Histological features such as glomeruli sclerosis, arteriolar hyalinosis, and the presence of inclusion bodies in the parietal epithelial cells, tubular epithelium, peritubular capillaries, and vessels were recorded as present or absent. Podocyte vacuolization was scored as none, mild (< 25%), moderate (25%-50%), and severe (> 50%). Podocyte inclusions were graded between 0 and 4+. Podocyte effacement was graded in percentage.
Serologic testing
Serum creatinine (SCr), eGFR, urine protein creatinine ratio, GL-3, and Lyso GL-3 enzyme activity, reported around the same time patients were biopsied, were collected. Renal function was assessed using serum creatinine and eGFR. Serum creatinine was reported as mg/dL, eGFR was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation and expressed as mL/min/1.73m2, and proteinuria detected by UPCR was defined as the ratio of random spot total protein to creatinine and expressed in mg/g.
Plasma GL-3 and Lyso GL-3 samples were collected in a heparin tube. Analysis was performed in one of two participating labs: the Genetic Testing Laboratory at the Department of Genetics and Genomics Sciences, Mount Sinai School of Medicine, and Genzyme, a division of Sanofi. Both plasma and Leukocyte enzyme activity were evaluated. GL-3 was extracted from the plasma with chloroform/methanol and purified by solid-phase chromatography. Total GL-3 was measured by liquid chromatography/tandem mass spectrometry. Plasma Lyso GL-3 was extracted from plasma by protein precipitation and lipid removal filtration. The extract was separated by reverse-phase liquid chromatography and Lyso GL-3 was quantified using a tandem mass spectrometer. The reference range for GL-3 was < 7.0 µg/mL, and the reference range for Plasma Lyso GL-3 was < 5.0 ng/mL.
Transthoracic resting echocardiogram for left ventricular hypertrophy
The size and pressure of the left and right ventricles and atria, the structure and function of the mitral, tricuspid, aortic, and pulmonary valves, and the root and dimensions of the great vessels were all assessed using MMode/2D measurements.
RESULTS
Thirteen adult female patients with confirmed Fabry disease diagnosis who underwent kidney biopsies at the University of Alabama at Birmingham between 2000 and 2020 were reviewed in retrospect. All thirteen participants were unrelated and had prior genetic testing to identify the α-galactosidase A gene mutation. The ages ranged between 17 and 48 years, and the mean age was 31.7 years. There was no prior history of hypertension or diabetes mellitus. Three patients (3, 9, and 10) had missense mutations, while others had classic nonsense mutations.
Acroparesthesia was present in 7 patients, hyperhidrosis in 2 patients, and gastrointestinal symptoms in 5 patients. Eye changes and angiokeratomas were present in 4 patients. The clinical characteristics of the 13 female Fabry disease patients are summarized in Table 1. At the time of kidney biopsy, all the patients had creatinine in the near normal range (reference range: 0.7-1.2 mg/dL). The median serum creatinine was 0.7 mg/dL (0.5-1.2). The eGFR was normal to mildly sub-normal and eGFR values were calculated by the
Genotype and clinical features of female patients with Fabry disease
| No. | Sex/Age (y) | Mutation category | Mutation | Acroparesthesia | Hyperhidrosis | Gastrointestinal symptoms | Eye changes | Angiokeratomas | LVH | ERT after kidney biopsy |
| 1 | F/46 | Classic nonsense | R227X | Y | N | Y | N | N | N | Y |
| 2 | F/35 | Classic missense | R112C | Y | N | Y | Y | Y | Y | Y |
| 3 | F/35 | Classic nonsense | Q221X | Y | N | N | N | N | N | Y |
| 4 | F/48 | Classic nonsense | W277X | - | N | - | - | - | - | - |
| 5 | F/38 | Classic nonsense | E79X | Y | N | Y | Y | Y | Y | Y |
| 6 | F/31 | Classic nonsense | R277X | N | Y | N | N | N | N | N |
| 7 | F/20 | Classic nonsense | R277X | Y | N | N | Y | N | N | Y |
| 8 | F/17 | Classic nonsense | W236X | N | N | N | Y | N | N | Y |
| 9 | F/24 | Classic missense | R227Q | N | Y | Y | - | N | - | - |
| 10 | F/39 | Classic missense | Y207S | Y | N | Y | N | Y | - | N |
| 11 | F/29 | Classic nonsense | W277X | N | N | N | N | N | N | Y |
| 12 | F/32 | Classic nonsense | R277X | Y | N | N | - | N | N | Y |
| 13 | F/18 | Classic nonsense | R277X | N | N | N | N | Y | N | Y |
The median UPCR was 0.16g/g (0.04-0.51). None of the patients were on Renin Angiotensin Aldosterone System inhibitors at the time of their biopsy. Plasma Lyso GL-3 was undetectable for the majority; three patients had detectable but minimally elevated Lyso GL-3 levels (reference range: < 5.0 ng/mL). GL-3 was below 3 µg/mL for all patients (reference range: < 7.0 µg/mL). Left ventricular hypertrophy was present in 2 patients and echocardiogram data were unavailable in 3 patients. Sixty-nine percent of the patients (9/13) were started on enzyme replacement therapy after the biopsy.
Kidney biopsy
On light microscopy, there was global glomerular sclerosis seen in 3 patients, involving 2 glomeruli in one patient and 1 glomerulus in two patients. There was minimal focal glomerular sclerosis seen on light microscopy in 1 patient. All the patients had enlarged parietal epithelial cells with a vacuolated appearance. Interstitial fibrosis and tubular atrophy (IFTA) is absent in all the patients. Minimal hyaline arteriopathy was seen in 61.5% (8/13 patients) and was absent in the others. The average score grading vacuolization of podocytes per the ISGFN was 2.2 (maximum score of 3); 38% of patients (4/13 patients) had ≥ 50% foot process effacement. The mean score of the biopsies was 2.43.
Figures 1 and 2. show the light microscopy. Figures 3 and 4 shows the electron microscopy of a female patient. The scoring sheet used in evaluating the biopsies can be found in the Supplementary Materials. The laboratory and the kidney biopsy findings are summarized in Table 2.
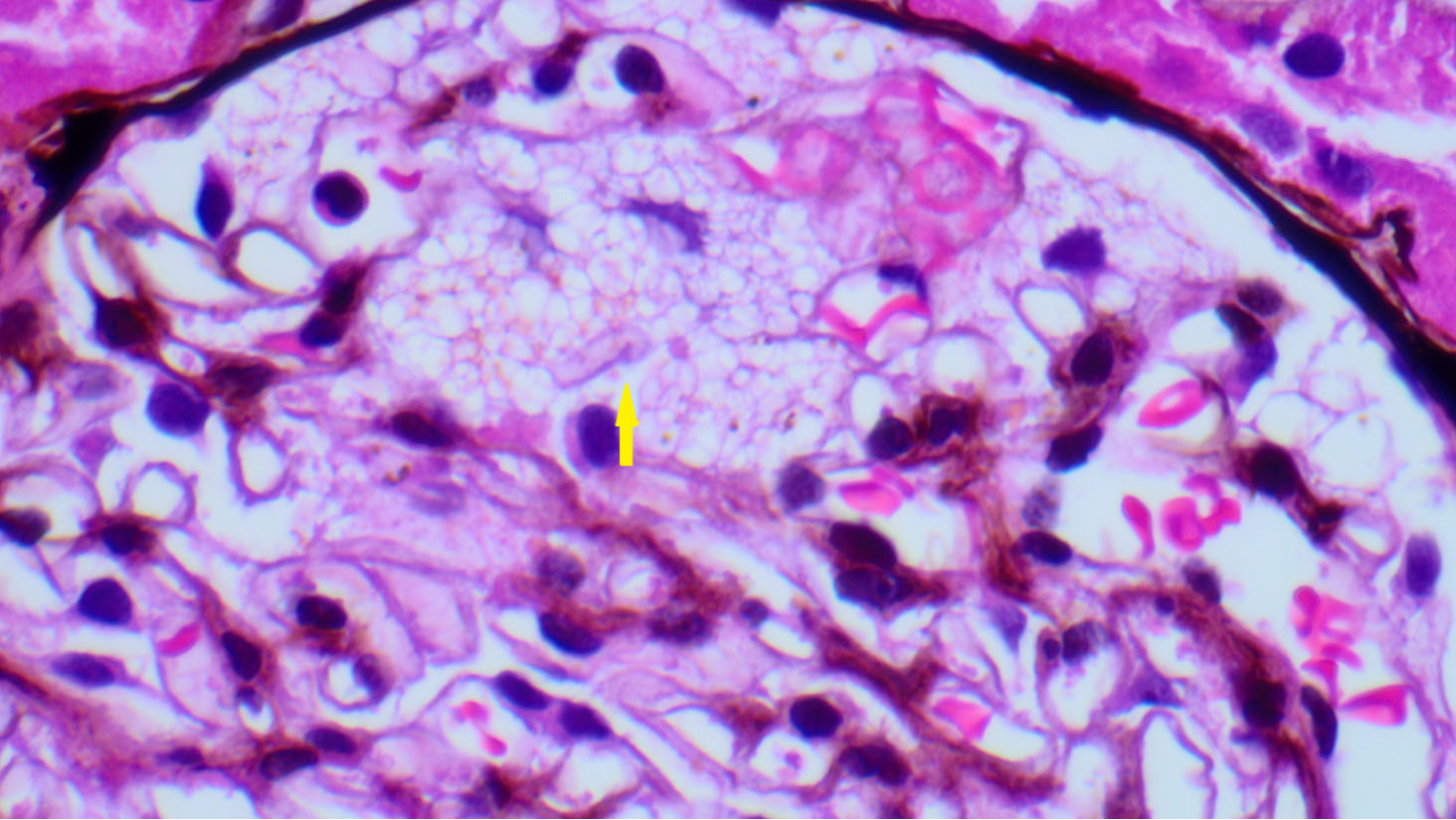
Figure 1. Light microscopy of the kidney biopsy of a female patient. The glomerulus shows swollen podocytes with finely vacuolated cytoplasm (yellow arrow) (H&E; x40).
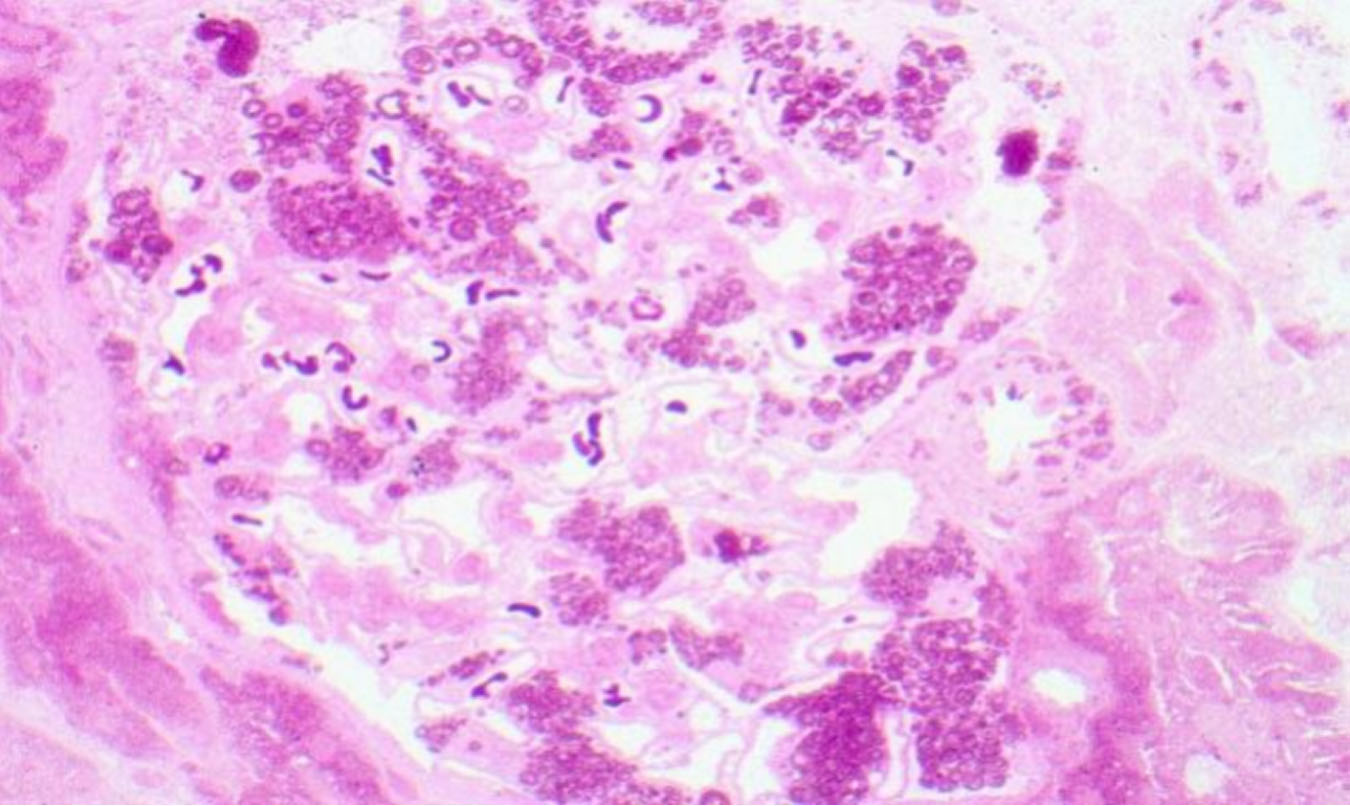
Figure 2. Light microscopy of the kidney biopsy of a female patient. Well-preserved intracellular lipid inclusions in osmicated,
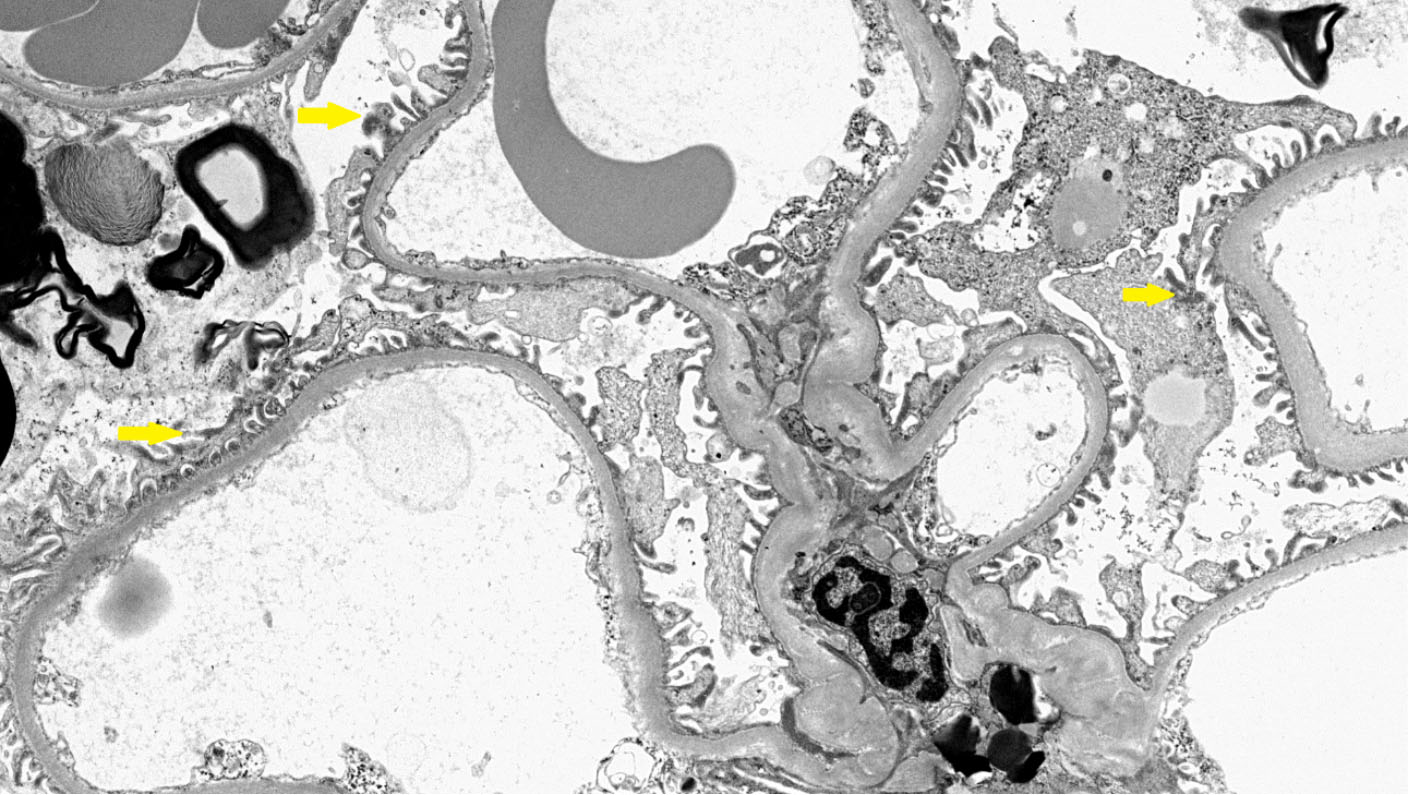
Figure 3. Electron microscopy of the kidney biopsy of a female patient. The glomerulus shows podocytes with normal foot processes (yellow arrows).
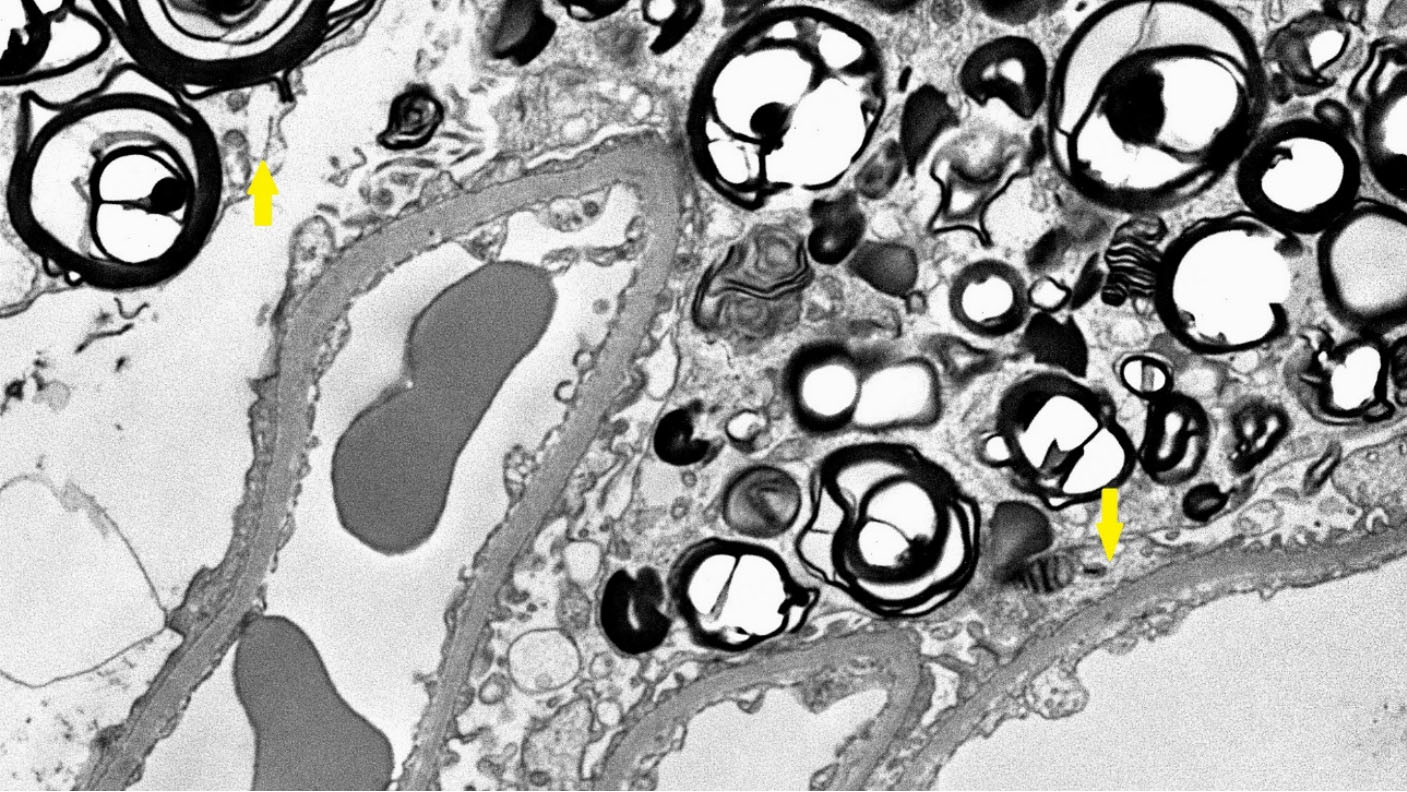
Figure 4. Electron microscopy of the kidney biopsy of a female patient. The glomerulus shows lamellated lipid inclusions (myeloid bodies) in podocytes (yellow arrows).
Baseline characteristics and kidney biopsy findings of female patients with Fabry disease at the time of kidney biopsy
| No. | SCr (mg/dL) | UPCR (g/g) | CKD EPI-eGFR (mL/min/1.73m2) | Lyso GL-3 (ng/mL) | Affected glomerular sclerosis (#) | Affected focal glomerular sclerosis (#) | Total points by scoring system | Podocyte inclusions | Effacement (%) |
| 1 | 0.6 | 0.33 | 106 | < 5.0 | N | N | 3 | Y | Suboptimal |
| 2 | 0.8 | 0.06 | 81 | 5.1 | N | N | 3 | Y | 30 |
| 3 | 0.7 | 0.04 | 93 | < 5.0 | 2 | N | 2 | Y | > 50 |
| 4 | 1.2 | 0.51 | 56 | < 5.0 | N | N | 3 | Y | < 50 |
| 5 | 0.6 | 0.23 | 108 | < 5.0 | N | N | 2 | Y | 50 |
| 6 | 0.8 | 0.16 | 83 | < 5.0 | N | N | - | Y | 30-50 |
| 7 | 0.7 | 0.06 | 90 | 2.3* | 1 | N | 2 | Y | 35 |
| 8 | 0.7 | 0.12 | 108 | < 5.0 | N | N | 2 | Y | 10 |
| 9 | 0.8 | 0.41 | 105 | < 5.0 | 1 | N | - | Y | 10 |
| 10 | 0.8 | 0.12 | 79 | 5.1 | N | N | 2.3 | Y | 50 |
| 11 | 0.5 | 0.16 | 130 | NA | N | 1 | 2 | Y | < 50 |
| 12 | 0.8 | < 0.08 | 100 | < 5.0 | N | N | 2 | Y | 75 |
| 13 | 0.7 | 0.26 | 130 | < 5.0 | N | N | - | Y | < 10 |
DISCUSSION
Our study shows that a significant number of female patients with FD with low Lyso GL-3, low levels of proteinuria, and creatinine values within the normal reference ranges have histological signs of the disease on renal biopsy. FD is characterized by the progressive intracellular accumulation of glycolipids, which resemble myelin primarily in podocytes. This gradual accumulation also occurs in the mesangial, glomerular, endothelial, tubular epithelial cells, and arteries[15]. Due to variations in the lyonization of the
Lyso GL-3 has been used as a biomarker to monitor disease activity and to monitor response to enzyme replacement therapy. However, it may be less reliable in females due to lower baseline Lyso GL-3 levels[16]. Due to these limitations, histology has been a mainstay for assessing FD involvement in oligosymptomatic female Fabry patients[17].
In our series, we demonstrate the presence of podocyte inclusions and foot process effacement, although the Lyso GL-3 levels were low, creatinine values were within the normal range, and proteinuria was minimal. This indicates that significant histologic damage occurs in females even with a normal or near normal baseline serologic evaluation. The standardized scoring system by Fogo et al. was developed using a modified Delphi technique combining the expertise of a panel of experts. It has been validated in female patients, and so we used it to grade the severity of histopathological features found on kidney biopsy. This scoring system grades podocyte vacuolization and inclusions based on the extent of involvement, while glomerular and interstitial lesions are graded based on severity. The minimum score is 0 and the maximum score is 3[13]. The average score using this scoring system among our series was 2.4, which was significant.
The pathognomonic osmiophilic, lamellar inclusion bodies are typically present within podocytes on electron microscopy. It has been found that increased amounts of GL-3 inclusions correlated with decreased podocyte numbers and a higher degree of proteinuria[18]. Podocytes in the female Fabry phenotype were found to have increased foot process width, and these dysfunctional podocytes are lost with age. Segmental flattening of the foot processes in young Fabry disease patients with classic disease has been described by Tøndel et al. in their case series. The occurrence of foot process effacement prior to the development of overt proteinuria is thought to be due to signaling pathways and local inflammatory processes in addition to the accumulation of GL-3[19].
Other non-electron microscopy findings are also of significance. Glomerular sclerosis occurs early in FD, and it has been reported that the presence of segmental and global glomerular sclerosis accelerates the eGFR decline[13,20]. As seen in other diseases, the presence of numerous glomerular sclerosis lesions portends a poorer prognosis. In our series, three patients had evidence of global sclerosis, and one had focal glomerular sclerosis. This again underscores that these findings occur before the onset of commonly identified signs of renal involvement, such as proteinuria or elevated creatinine. Interstitial fibrosis has also been associated with poorer prognosis in Fabry, similar to IgA nephropathy and other diseases. A small study FD study involving nine children and adolescents, with an average age of 13.5 years and minimal albuminuria, exhibited biopsy changes, including interstitial fibrosis and arteriopathy, indicating that these processes start early[21]. This was also seen in our study. Although IFTA was minimal among our patients, it was present in the majority. There are no reported gender dissimilarities in biopsy findings, but males have been reported to have larger and increased podocyte inclusion bodies involving > 50% of the glomerulus even in the early stages of CKD. Generally, females may have fewer histopathological features than males, such as global sclerosis, podocyte vacuolization, and vascular inclusions[13].
According to data from the Fabry registry, the most common initial heralding events are renal failure and cardiac arrhythmias. Approximately 20% of females over the age of 40 years have stages 3-5 CKD. It must be noted that renal disease can contribute to and result from cardiac disease. The mean ages of death per the Fabry registry and the Fabry outcomes survey (FOS) are 64.4 and 60.9 years, respectively, which are lower than those of the general population. Early diagnosis and early implementation of therapies can have a positive impact by preventing organ damage and improving the overall quality of life[22].
Classic mutations in FD lead to a complete absence of GLA, which is associated with more severe manifestations of the disease[23]. While classic mutations typically cause a more aggressive disease course in males, female patients with these mutations can also exhibit severe phenotypes due to skewed
Endomyocardial biopsies also play a role in assessing the extent of tissue involvement in patients with FD, as well as in cases with variants of uncertain significance (VUS). In regions such as Taiwan, where biopsies are mandatory to verify the primary etiology of hypertrophic cardiomyopathy, endomyocardial biopsies are performed more frequently. Hsu et al. have shown that endomyocardial biopsies among Taiwanese patients with IVS4 + 919G > A, a late-onset mutation, showed GL-3 accumulation within cardiomyocytes but not in the capillary endothelial cells. The median creatinine among females in the cohort was 0.8 mg/dL and the mean eGFR calculated per the MDRD equation was 73.2 mL/min/1.73m2[24]. This suggests that the females in the cohort may have renal involvement. Kidney biopsies are therefore helpful in such cases, as they not only confirm the presence of GL-3 accumulation, but also assess the extent of its impact on other structures, such as podocytes, in addition to demonstrating IFTA and other structural changes.
Although kidney biopsies are valuable in establishing a baseline in FD, females with classic symptoms such as angiokeratomas or elevated Lyso GL-3 may not require one. We recommend that in cases with positive biomarkers, the necessity of a kidney biopsy should be carefully considered and treatment regimen should be tailored with the help of experienced nephrologists. Based on our experience, we also recommend that in the presence of a significant decline in eGFR > 3 mL/min/1.73m2 per year, a kidney biopsy should be considered to evaluate other underlying pathologies.
Currently, three different enzyme replacement therapies (ERTs) and one pharmacological chaperone are approved for the treatment of FD[25,26]. As more therapeutic options become available, we believe that kidney biopsies may play a crucial role in guiding treatment choices. In such cases, a baseline kidney biopsy could be performed prior to treatment initiation, with a follow-up biopsy after 3-4 years to evaluate the need for adjustments in therapy.
Our study has some limitations. Although we report low Lyso GL-3 levels, at the time of data collection, the assay for Lyso GL-3 detection had a reference cut-off level of <5 ng/mL. The newer assays currently used can detect lower levels of Lyso GL-3 of > 0.3 ng/mL. It is possible that the patients in our series with <
In conclusion, we report the histopathological findings on the kidney biopsy of 13 female patients with a known diagnosis of FD but with serum creatinine within the normal reference range and low plasma Lyso GL-3. The patients in our series also had minimal proteinuria < 0.5 g/g, despite having evidence of podocyte inclusion bodies and varying degrees of foot process effacement. These findings suggest that kidney biopsy of female patients with normal serologic work-up and who are oligosymptomatic may still have significant histologic damage. Kidney biopsies in this population can help guide clinical management.
DECLARATIONS
Authors’ contributions
Conceptualization: Wallace E, Warnock DG, Chandramohan D, Moreman M, Madi S
Data curation: Ahmad M, Huma F, Singleton C, Wallace E
Methodology, investigation: Chandramohan D, Moreman M, Madi S, Huma F, Ahmad M, Singleton C, Warnock DG
Writing, review, and editing: Chandramohan D, Moreman M, Madi S, Wallace E
Supervision: Warnock DG, Wallace E
All authors have read and agreed to the final version of the manuscript.
Availability of data and materials
The data are available from the corresponding author upon reasonable request.
Financial support and sponsorship
The authors received no financial support for this article's research, authorship, and/or publication.
Conflicts of interest
Dr. Wallace E has received research funding from Chiesi, Sanofi Genzyme, Uniqure, Spark Therapeutics, and Idorsia, consulting fees from Chiesi, Sanofi Genzyme, and Kyowa Kirin, and speaker honoraria from Chiesi, Sanofi Genzyme, Natera, and Amicus. Dr. Warnock DG has received consulting fees from Amicus, Chiesi, Idorsia, and Protalix. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The institution Review Board at the University of Alabama in Birmingham reviewed and approved the study protocol (IRB-300004655). Written informed consents were obtained prior to the kidney biopsies, but consent for the study was waived due to the minimal risk nature of the project.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
2. Lukas J, Giese AK, Markoff A, et al. Functional characterisation of alpha-galactosidase a mutations as a basis for a new classification system in Fabry disease. PLoS Genet. 2013;9:e1003632.
3. Eng CM, Fletcher J, Wilcox WR, et al. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the fabry registry. J Inherit Metab Dis. 2007;30:184-92.
4. Galanos J, Nicholls K, Grigg L, Kiers L, Crawford A, Becker G. Clinical features of Fabry's disease in Australian patients. Intern Med J. 2002;32:575-84.
5. Gupta S, Ries M, Kotsopoulos S, Schiffmann R. The relationship of vascular glycolipid storage to clinical manifestations of Fabry disease: a cross-sectional study of a large cohort of clinically affected heterozygous women. Medicine. 2005;84:261-8.
6. Orteu CH, Jansen T, Lidove O, et al. FOS Investigators. Fabry disease and the skin: data from FOS, the Fabry outcome survey. Br J Dermatol. 2007;157:331-7.
7. Echevarria L, Benistan K, Toussaint A, et al. X-chromosome inactivation in female patients with Fabry disease. Clin Genet. 2016;89:44-54.
8. Merscher S, Fornoni A. Podocyte pathology and nephropathy - sphingolipids in glomerular diseases. Front Endocrinol. 2014;5:127.
9. Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348-54.
10. Kilbride HS, Stevens PE, Eaglestone G, et al. Accuracy of the MDRD (modification of diet in renal disease) study and CKD-EPI (CKD epidemiology collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61:57-66.
11. Sakuraba H, Togawa T, Tsukimura T, Kato H. Plasma lyso-Gb3: a biomarker for monitoring fabry patients during enzyme replacement therapy. Clin Exp Nephrol. 2018;22:843-9.
12. Young E, Mills K, Morris P, et al. Is globotriaosylceramide a useful biomarker in Fabry disease? Acta Paediatr Suppl. 2005;94:51-4; discussion 37.
13. Fogo AB, Bostad L, Svarstad E, et al. all members of the International Study Group of Fabry Nephropathy (ISGFN). Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant. 2010;25:2168-77.
14. Valbuena C, Carvalho E, Bustorff M, et al. Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch. 2008;453:329-38.
15. Fischer EG, Moore MJ, Lager DJ. Fabry disease: a morphologic study of 11 cases. Mod Pathol. 2006;19:1295-301.
16. Germain DP, Altarescu G, Barriales-Villa R, et al. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol Genet Metab. 2022;137:49-61.
17. van der Tol L, Svarstad E, Ortiz A, et al. Chronic kidney disease and an uncertain diagnosis of Fabry disease: approach to a correct diagnosis. Mol Genet Metab. 2015;114:242-7.
18. Najafian B, Silvestroni A, Sokolovskiy A, et al. A novel unbiased method reveals progressive podocyte globotriaosylceramide accumulation and loss with age in females with Fabry disease. Kidney Int. 2022;102:173-82.
19. Tøndel C, Kanai T, Larsen KK, et al. Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron. 2015;129:16-21.
20. Rusu EE, Zilisteanu DS, Ciobotaru LM, et al. The impact of kidney biopsy for Fabry nephropathy evaluation on patients' management and long-term outcomes: experience of a single center. Biomedicines. 2022;10:1520.
21. Tøndel C, Bostad L, Hirth A, Svarstad E. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767-76.
22. Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry registry. Genet Med. 2009;11:790-6.
23. Burlina A, Brand E, Hughes D, et al. An expert consensus on the recommendations for the use of biomarkers in Fabry disease. Mol Genet Metab. 2023;139:107585.
24. Hsu TR, Chang FP, Chu TH, et al. Correlations between endomyocardial biopsies and cardiac manifestations in taiwanese patients with the Chinese hotspot IVS4+919G>A mutation: data from the Fabry outcome survey. Int J Mol Sci. 2017;18:119.
25. Umer M, Kalra DK. Treatment of Fabry disease: established and emerging therapies. Pharmaceuticals. 2023;16:320.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].