MOF and related composites as selective functional separators and interlayers for Li-S batteries
Abstract
Lithium-sulfur (Li-S) batteries are one of the most promising technologies compared to lithium-ion-based ones, mainly due to their outstanding high energy density (2,567 Wh/kg). Nonetheless, Li-S batteries still face important drawbacks, namely the shuttle effect caused by the polysulfide dissolution into the electrolyte and their escape from the cathode, leading to active material loss and ultimately to the anode passivation. Mitigating this effect is crucial to boost the Li-S technologies at a large scale and the rational design of the separator or interlayer is considered as an effective solution. Metal-Organic Frameworks and related composites have been recently proposed as candidates to selectively capture the polysulfides, due to their tunable structures and compositions and ordered micro- or meso-porosity which can sieve polysulfides through physical barriers or chemical sorption and catalyze polysulfide conversion kinetics. Moreover, once introduced into composite membranes as functional separators and interlayers, this promotes their easy inclusion in Li-S devices. This short review summarizes the recent progress in this field, emphasizing the different types of functional separators and interlayers integrating Metal-Organic Frameworks, and proposes new research directions to optimize these systems.
Keywords
INTRODUCTION
Over the last decades, sustainable energy storage has become one of the biggest societal challenges. Lithium (Li)-ion batteries have bloomed in this landscape, becoming one of the major actors in this field, from small electronic devices up to electric vehicles[1-5]. Nonetheless, they are reaching their limits in terms of energy density and more efficient and sustainable next-generation energy storage solutions are needed. Lithium-sulfur (Li-S) batteries are among the greatest candidates to meet these criteria, since sulfur is abundant, non-toxic, and inexpensive, resulting in a high energy density system compared to LiCoO2 for instance (2,567 vs. ≈ 200 Wh kg-1)[6-9]. Moreover, recent life cycle assessments of the whole aspects of Li-S technologies applied to electromobility, from efficiency under operating to recycling, clearly demonstrate a promising electrochemical energy storage system within our reach[10,11]. Despite numerous advantages, important drawbacks still hinder their development, and their commercialization remains limited until these shortcomings and issues of the related materials are not well understood and solved[12-16]. Indeed, during reduction, sulfur forms polysulfide chains Li2Sx (2 ≥ x ≥ 8) through a complex multi-electron conversion mechanism. These polysulfides are soluble in the electrolyte and migrate toward the anode. This phenomenon, known as the shuttle effect, simultaneously leads to anode passivation, loss of active material, poor cyclability and low coulombic efficiency upon long-term cycling[17-19].
To mitigate this unwanted effect, porous interlayers or coated separators have been developed to sieve the soluble species[20-23]. Usually, these are made of porous carbon materials (e.g., microporous carbon or mesoporous carbon)[24-29]. However, their efficiency is often limited due to several intrinsic drawbacks. The relatively large and uneven pore sizes of these materials fail to act as effective physical barriers to block polysulfides. Furthermore, the low affinity between polysulfides and the membrane surface reduces their ability to restrict polysulfide migration. Hence, new ordered and functional materials with high structural tunability and advanced physico-chemical properties are needed to overcome the shuttle effect limitations. Metal-Organic Frameworks (MOFs) appear as ideal materials for the selective capture of polysulfides, since their porous crystalline framework can be easily tuned to achieve a desired property through the combination of the appropriate organic and inorganic building units[30,31]. This results in a large number of structures with uniform micro or mesopores (channels and/or cages), bearing a large diversity of functionalities (Lewis or Brönsted acid/basic sites, and hydrophobic or hydrophilic groups)[32-34]. MOFs have recently received increased interest for energy-related applications, in particular in the Li-S batteries field[35-38], and MOF-based membranes have been highlighted as promising solutions to mitigate the shuttle effect.
However, although the number of reviews about MOFs and derived composites as functional separators and interlayers for Li-S devices has increased in the last five years[39-42], several points remain unclear: what are (1) the key structural features which the MOF host matrix should display (pores size and shape, open metal sites, structural defects); and (2) the size and morphology of MOF particles and how this influences the mechanical stability of the films.
Herein, we will discuss the main features of Li-S systems, while proposing a distinction between modified functional separators and interlayers and the use of pristine MOFs and related composites in these architectures. We propose a broad analysis of the state of the art, highlighting the different categories of separators, with the exception of MOF-derived carbon compounds. These composites, derived from MOF pyrolysis, are the object of complementary reviews and will not be covered here[43-46]. Finally, we suggest potential future research directions for enhancing the performance of MOF-based composites as efficient interlayers and separators for Li-S batteries.
LITHIUM-SULFUR BATTERIES: BASIC PRINCIPLES
Nowadays, seeking energy systems based on abundant and sustainable elements is one of the major trends in the energy landscape. Despite the use of lithium metal, whose abundance and extraction are major drawbacks, Li-S devices are particularly interesting since sulfur is naturally abundant and environmentally friendly. Notably, sulfur availability can be considered unlimited, since its origin comes from volcanic activity, but today, this chemical element is recovered as a major by-product of oil refining and natural gas purification[47]. The high theoretical specific capacity (1,675 mA h g-1) and energy density (2,567 Wh.kg-1)[9]. of Li-S systems result from the sulfur reduction, through a 16 e- mechanism and following
The architecture of Li-S batteries is quite similar to traditional Li-ion batteries. It consists of a positive electrode, generally a porous carbon with a high sulfur lading embedded; liquid organic electrolytes with lithium salts; a separator between the two electrodes and lithium metal as a negative electrode [Figure 1A]. The initial state of sulfur is S8 (cyclic octasulfur), and during the reduction process (from 0 to -2 state), sulfur reacts with Li+ ions and undergoes compositional and structural changes, with the formation of the polysulfide intermediates. The sulfur redox reaction is extremely complex, as initially, sulfur forms chains known as polysulfides (Li2Sx, with 2 ≥ x ≥ 8). The first stage of the discharge process occurs at ~2.3 V (vs.
which quickly evolves to Li2S6 and Li2S4 [Figure 1B] through
In the second stage of the discharge, lithium polysulfides further reduce to insoluble Li2S2/Li2S through
at the average voltage of ~2.1 V (vs. Li/Li-)[36,49]. When the Li-S battery is charged, the opposite process occurs, starting from Li2S and ideally all lithium polysulfides are reconverted to elemental sulfur.
Despite these promising features, one of the most critical issues is the polysulfide shuttle effect. During the discharging process, the redox intermediates long chain polysulfides are extremely easy to dissolve in the ether-based electrolyte and migrate through the traditional separator and interact with lithium anode to yield insoluble Li2S2/Li2S. As the discharge process continues, a passivation layer of insoluble Li2S2/Li2S is formed and covers the anode surface. When the charging process in Li-S batteries occurs, the oxidation of the insoluble Li2S2/Li2S layer into polysulfides is not favored, which leads to irreversible loss of active materials. Furthermore, as the charge-discharge reaction cycle number increases, the non-conductive
MOF-BASED SEPARATORS AND INTERLAYERS
When talking about Li-S systems, functional separators or interlayers are often mentioned with no distinction. However, it should be clarified that these two types of engineering are distinct architectures, as depicted in Figure 2.
Figure 2. Schematic diagram of the difference between the configuration of (A) functional interlayers and (B) functional separators for Li-S batteries. (C) New classification in terms of the functions in the form of the interlayer/separator for Li-S batteries. Reproduced with permission[20]. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Although Huang et al. proposed some time ago a clear distinction between the two concepts, there are until now still no distinctions between these two types of films in most MOF-related published works[22]. An interlayer is usually a self-standing film, placed between the sulfur cathode and the common separator [Figure 2A], while a functional separator is a routine separator (Celgard or glass fiber (GF) usually) which has been modified [Figure 2B], by adding a functional layer in one or both sides of the separator. The efficiency of both interlayers and functional separators relies on the equilibrium between three key aspects
Advantages and disadvantages of functional separators and interlayers
| Aspect | Functional separators | Interlayers |
| Advantages | Suppress polysulfide diffusion by integrating MOFs or composites | Highly effective polysulfide trapping and catalytic conversion, improving sulfur utilization |
| Simple design and easy integration into existing separators | Enhances redox kinetics and reduces the shuttle effect | |
| Improves electrolyte wettability with functionalized materials | Acts as an additional host for sulfur, enabling higher sulfur loading and capacity | |
| Scalable and compatible with current separator manufacturing processes | Provides a dedicated platform for catalytic conversion of polysulfides | |
| Disadvantages | Limited surface area for polysulfide conversion compared to interlayers | Adds weight and volume to the battery, potentially lowering energy density |
| Increased separator thickness and resistance can hinder ionic conductivity | Requires additional fabrication steps, increasing complexity and cost | |
| Coating adhesion must be robust to prevent delamination during cycling | Poorly designed interlayers may introduce unwanted side reactions or increase internal resistance | |
| Reduced catalytic effect compared to interlayers, depending on the MOF or composite material used | Integration with separators and cathodes must be optimized to ensure compatibility and mechanical stability |
Although numerous improvements have been proposed in preventing the polysulfide shuttle effect, in particular by developing PS-function-based materials, it remains challenging to combine within the same material the perfect equilibrium of the three components. In this landscape, MOFs appear as great candidates due to their remarkable structural versatility [Figure 3].
Figure 3. Examples of benchmark MOFs: (A) MIL-53[50]; © 2010, Springer Nature. (B) UiO-66; (C) HKUST-1 and (D) ZIF-8;
One key feature of MOFs is their highly ordered structure and intrinsic porosity, which encompasses micro- or meso-pores of different sizes and shapes, or even a hierarchical arrangement of both. The structural characteristics are associated with high surface area and efficient sieving and transport within the material[52,53]. In the usual 1,3-dioxolane (DOL)/dimethoxy ethane (DME) electrolyte, polysulfides range in size from approximately 0.81 to 1.92 nm, while lithium ions are smaller, ranging from 0.64 to 1.48 nm[54]. Thus, microporous MOFs with pore sizes below 2 nm are expected to be highly effective in order to trap small polysulfides due to size exclusion but may also lead to a reduction of the ionic conductivity, leading to increased polarization and reduced performance[55]. On the other hand, mesoporous MOFs (mostly with pore sizes between 2 and 5 nm) can balance polysulfide trapping and ionic conductivity, though larger pore sizes are likely to increase the polysulfide shuttle effect. Hence, the pore size/polarity of MOFs must be carefully selected to achieve an optimal balance. Another advantage of MOFs is their hybrid nature, comprising both organic linkers and inorganic sub-units. This, together with the control of the pore architecture, enables a precise tuning of the selectivity of MOFs. By further functionalizing the organic linkers with polar functional groups, the interaction between the MOF and polysulfides can even be enhanced, promoting their immobilization and minimizing their shuttle effect[56]. Typically, organic linkers bearing polar functional groups, such as -NH2, -COOH, or -SO3H, can enhance the interactions with lithium polysulfides through hydrogen bonding, electrostatic forces, or dipole interactions[57,58]. Specifically, -NH2 offers strong Lewis acid-base interactions with high polysulfide binding affinity while -COOH provides balanced adsorption through dual-site interactions (C=O coordination and -OH hydrogen bonding) and -SO3H enhances polysulfide trapping via strong hydrogen bonding and electrostatic interactions while improving ionic transport. These functional groups are expected to enhance the affinity of MOFs for polysulfides, effectively immobilizing them within the cathode region. The inorganic sub-units of MOFs, typically featuring Lewis or Brønsted acidic/basic sites, also play a crucial role in capturing and stabilizing polysulfides in Li-S batteries. Open metal sites, such as unsaturated Cu or Fe nodes, act as open metal sites that effectively bind negatively charged polysulfide anions (Sn2-)[59]. This interaction prevents the diffusion of polysulfides into the electrolyte, thereby mitigating the polysulfide shuttle effect and improving battery stability. However, these strong sulfur-metal interactions can lead to the formation of metal-polysulfide complexes (e.g., metal-Sx2-), resulting in a slight initial loss of active sulfur and a reduction in initial capacity. Beyond their trapping ability, these inorganic sub-units also serve as catalytic sites, facilitating the electrochemical reduction of high-order polysulfides (e.g., Li2S8) into lower-order species (e.g., Li2S4 and Li2S), which significantly enhance reaction kinetics and boost the overall performance of the battery[43,60,61]. On the other hand, metals such as zinc (Zn), magnesium (Mg), and aluminum (Al) lack intrinsic catalytic activity but provide exceptional structural stability and durability to the MOF, ensuring reliable performance over prolonged cycling. Furthermore, these metals contribute to scalability and cost-effectiveness, making them highly attractive for the practical commercialization of MOF-based Li-S batteries. Table 2 proposes an overview of the key structural features of MOFs.
Key structural features of MOFs for enhanced capture and efficient catalytic conversion of polysulfides
| Aspect | Key insights | |
| Pore sizes | Microporous (< 2 nm) | Traps small polysulfides but may restrict ion transport |
| Mesoporous (optimal | Balances trapping and ion conductivity | |
| Functional groups | NH2 (Amine) | Strong Lewis acid-base interactions dominate, offering a high binding affinity for polysulfides |
| COOH (Carboxyl) | Relies on dual-site interactions (C=O for coordination and -OH for hydrogen bonding), providing balanced adsorption | |
| SO3H (Sulfonic), highly acidic | Focuses on strong hydrogen bonding and electrostatic interactions, with added benefits for ionic transport | |
| Metal element | Catalytic metals (e.g., Fe, Co, Ti) | Enhance polysulfide trapping and conversion, while the metal-SX2- causes the initial loss of S |
| Stable metals (e.g., Zn, Mg, Al) | Provide structural stability but limited catalytic properties | |
| Lightweight and abundant metals | Reduce cost while maintaining functionality | |
Figure 4 gathers the main structural features of MOFs contributing to an effective adsorption and catalytic conversion of the polysulfide species. By leveraging the unique structure and hybrid composition of MOFs, it becomes possible to design interlayers with tailored properties that effectively address the challenges associated with Li-S batteries, such as the migration of polysulfides and capacity fading.
The combination of polysulfide sieving (PS) and e-function materials is often described in literature, with carbon being the most common material to ensure good electronic activity and PS being ensured by different metal oxide particles. Two main strategies are used to combine the different components: (A) the layer-by-layer superposition of PS and e materials; (B) the immobilization of PS function particles within carbon sources. Most MOF-based separators are layer-by-layer architectures and will be detailed in the following sections. It should however be pointed out that the vast majority of MOFs display an (electronically) insulating character, and therefore the addition of a carbon layer is often required, except for conductive MOFs[62].
Finally, the sluggish kinetics of polysulfide redox reaction requires the use of species to catalyze the reaction, known as the k function. Numerous studies describe the use of catalysts to enhance the long-order polysulfides to shorter chains. Even though the catalytic effect of MOFs is mostly evoked when dealing with cathode architectures, one can expect the acid sites in MOF structures to play an important role in polysulfide conversion.
Overall, selected MOFs and related composites can combine into a single porous material with the three essential functions required to limit the shuttle effect:
• A well-ordered microporous structure with a selective sieving character, allowing the anchoring of the polysulfide species and therefore mitigation of the active material loss;
• A catalytic activity of the metal nodes, ensuring a convenient reversible redox reaction of sulfur;
• An enhanced electronic conductivity when associated with carbon, or through a specific design of its chemical features.
The upcoming sections explore the different categories of MOFs-based separators and interlayers, highlighting some of the key features (pores sizes, polar sites, structural dimensionality) and the current limitations in the field of MOFs for the selective sieving of polysulfides. Scheme 1 gathers and classifies the main categories of MOFs-based films or membranes which will be described hereafter.
MOF-based modified separators
Modified separators have been extensively reported by adding a thin layer of a functional material to sieve or selectively capture the polysulfides[63-65]. A typical strategy consists of constructing Janus membranes[66], an asymmetric composite, where each side of the membrane displays a different or sometimes even opposite character[67,68]. Such composites can be prepared either by coating the separator or growing MOF particles directly onto the separator surface; alternatively, one can carry out successive filtration to prepare MOF-based modified separators. The most common architectures consist of a layer of pristine MOF (nano) particles deposited onto a standard separator (polypropylene (PP) or GF), while on the other side, a layer of carbon can be found [Figure 5A]. In these composites, MOFs ensure a selective barrier with good mechanical stability due to the presence of the separator, while the carbon guarantees the required electric conductivity to reactivate polysulfide redox. Table 3 gathers the composites described in literature. In a general way, one can notice that, at a few exceptions, only benchmark MOFs have been considered to shape modified separators so far. According to the studies reported so far, the structural features, the presence of defects, Lewis acid sites, linker functional groups, bimetallic centers, and electrical conductivity of MOFs are of great significance in improving the performance of the modified separator. Besides the MOF structural types, one can also emphasize the key features which can infleunce the overall performance of the Li-S cell: (i) carbon type and wt%; (ii) shaping method; (iii) functional layer thickness; (iv) sulfur loading. Different types of carbons have been considered [carbon nanotubes (CNTs), graphene, super P, Ketjenblack (KB)] and with different loadings (from 10% up to 80%). Nonetheless, it is not possible to draw a clear link between the type of carbon and the performance of the cell. Furthermore, the two main shaping methods considered (casting and filtration) can both provide layers with thicknesses comprised between 8 and 20 µm (with some rare exceptions and very thin layers for conductive MOFs). Such results suggest that no clear trend can be drawn from simply considering the carbon type and amount, the shaping method or the functional layer thickness. Among these parameters, only the sulfur loading seems to have an impact; however, when looking carefully, one can see that the sulfur loading cannot be linearly connected to the capacity since even with an equivalent loading, functional layers containing MOFs with different structural features display different capacity values.
Characteristics and electrochemical performance of MOF-based modified separators
| MOF (wt%) | Carbon source (wt%) | Separator | Shaping | Thickness (µm) | Coating density (mg cm-2) | Sulfur loading (mg cm-2) | Specific capacity (mAh g-1) | Areal capacity (mAh cm-2) | Cycle number and cycling rate | Ref. |
| Y-FTZB (80) | CNT (20) | GF | Casting | 14 | 1.50 | 1.0 | 557 | 0.557 | 300 cycles at 0.25 C | [67] |
| ZIF-7 (80) | 14 | 1.50 | 1.0 | 452 | 0.452 | |||||
| HKUST-1 (80) | 14 | 1.50 | 1.0 | 197 | 0.197 | |||||
| ZIF-8 (80) | CNT (20) | GF | Casting | 14 | 1.50 | 1.0 | 403 | 0.403 | 300 cycles at 0.25 C | [67] |
| ZIF-8 (29.1) | CNT (67.9) | PP | Casting | 15 | 0.90 | 1.2 | 870 | 1.044 | 100 cycles at 0.2 C | [69] |
| ZIF-8 (20) | KB (80) | PP | Filtration | 20 | 0.06 | 1.2 | 606 | 0.727 | 100 cycles at 0.1 C | [70] |
| Fe-ZIF-8 (75) | Super P (15) | PP | Casting | 8 | - | 1.0 | 409 | 0.409 | 1,000 cycles at 0.5 C | [71] |
| UiO-66(Zr) (100) | - | PP | Filtration | 2 | - | 2.5 | 964 | 2.410 | 200 cycles at 0.5 C | [63] |
| UiO-66(Zr) (70) | Super P (20) | PP | Casting | 20 | 0.35 | 1.5 | 586 | 0.879 | 500 cycles at 0.5 C | [72] |
| UiO-66(Zr) (80) | Super P (10) | PP | Casting | - | 0.50 | 2.0 | 785 | 1.570 | 500 cycles at 1 C | [73] |
| UiO-66(Zr)-NH2 (70) | Super P (20) | PP | Casting | 5 | 0.42 | 2.0 | 855 | 1.710 | 70 cycles 0.1 C | [74] |
| UiO-66(Zr)-NH2 (75) | Graphene (25) | GF | Filtration | 1.5 | 0.30 | 1.4 | 502 | 0.703 | 500 cycles at 1 C | [75] |
| UiO-66(Zr)-NH2 (75) | Graphene (25) | GF | Filtration | 1.5 | 0.30 | 3.7 | 980 | 3.626 | 100 cycles at 0.1 C | [75] |
| UiO-66(Zr)-(SO3Li)4 (70) | Super P (20) | PP | Casting | 10 | 0.540 | 1.0 | 457 | 0.457 | 1,000 cycles at 1 C | [76] |
| MOF-808(Ce) (-) | CNT (-) | PP | Filtration | 8 | 0.40 | 2.5 | 838 | 2.095 | 800 cycles at 1 C | [77] |
| Cu2(CuTCP) (93) | - | PP | Filtration | 0.5 | 0.10 | 2.0 | 1020 | 2.040 | 100 cycles at 0.2 C | [78] |
| Ni3(HITP)2 (100) | - | PP | Interface-induced growth | 0.34 | - | 3.5 | 1139 | 3.987 | 100 cycles at 0.2 C | [64] |
| Ni3(HITP)2 (100) | - | PP | Filtration | 8 | 0.33 | - | 600 | - | 200 cycles at 0.5 C | [79] |
| Zn-Co MOF (80) | KB (10) | PP | Casting | 9 | 0.80 | 5.5 | - | 5.000 | 100 cycles at 0.1 C | [80] |
Li et al. described the use of Cu- or Zn-based MOFs with different particle morphologies and pore sizes, namely ZIF-7, a Zn-benzimidazolate MOF with 2.9 Å pores[67]; ZIF-8, a Zn-2-methylimidazolate MOF with 3.4 Å pores; Y-FTZB, featuring 6 Å pores and composed of [Y6(μ3-OH)8]10+ clusters coordinated with
Figure 5. (A) Schematic for the lithium-sulfur battery configuration consisting of a sulfur cathode, a MOF/CNTs modified separator, and an anode of lithium. SEM images of the stacking structure of the (B) Y-FTZB, (C) ZIF-7, (D) ZIF-8, and (E) HKUST-1 separators. The scale bar is 200 nm. Reproduced with permission[67]. © 2017, American Chemical Society. (F) Structure, metal nodes, and the Li2S6 adsorption sites in the Ce-MOF-808. Reproduced with permission[77]. © 2019, American Chemical Society.
Although all MOFs displayed different structural features and chemical natures, the authors emphasized that smaller pore sizes do not necessarily result in more effective polysulfide inhibition. They raised instead the role of the packing density, proposing that the Li-S battery modified with Y-FTZB on the GF separator exhibited the best performance with a capacity of 557 mAh g-1 after 300 cycles at 0.25 C due to its highest packing density. Albeit this feature certainly plays an important role as raised by the authors, this study remains quite limited as MOFs with very different structural features and particle size and morphology are compared. Moreover, the electrochemical stability of the MOFs should also be considered. The microporous hydrophobic ZIF-8 has been further reported as an efficient compound for polysulfide shuttling mitigation due to its enhanced chemisorption of lithium polysulfides[69,70]. Wu et al. proposed CNT@ZIF-8 composites, where the use of electronically conductive multiwalled CNTs (MWCNT) not only increased the physical adsorption of Li2Sx but also enhanced their reactivation by acting as a secondary current collector, in synergy with the adsorption capacity of the ZIF-8[69]. The Li-S cell, based on their best composite, containing 30% of ZIF-8, delivered a specific capacity of 870 mAh g-1 after 100 cycles at
One feature of selected MOFs is their ability to contain open metal sites, either as part of their structure or as missing ligands or inorganic node defects. This feature enhances the adsorption of the polysulfides and their catalytic conversion. For instance, UiO-66(Zr)-modified separators have been frequently reported as containing a high content of defects (linkers and clusters). UiO-66(Zr) is composed of Zr6O4(OH)4 clusters interconnected by 1,4-benzenedicarboxylate (BDC) ligands, forming a stable three-dimensional framework. Due to variable degrees of defect content, the pore sizes could vary widely, ranging from approximately 5 to 18 Å[73,81]. These defects have been reported to boost the conversion of polysulfides and inhibit the shuttle effect. Surprisingly, the modified separators with pristine UiO-66(Zr) proposed by Han et al.[63] displayed higher performances compared to the composites containing a carbon moiety proposed by Fan et al.[72] and Wang et al.[73] respectively displaying capacities of 964 mAh g-1 (200 cycles at 0.5 C), 586 mAh g-1 (500 cycles at 0.5 C), and 785 mAh g-1 (500 cycles at 1.0 C). In comparison, the original commercial PP separator only delivered 320 mAh g-1 after 500 cycles at 0.5 C[72]. Besides the reduced shuttling due to the chemical adsorption and catalytic conversion of polysulfides into defective micropores, the role of defects in inhibiting the migration of anions through electrostatic adsorption and increasing the Li+ transference number was also evidenced[73]. Besides this effect, the physical barrier at grain boundaries between the MOF nanoparticles (~400 nm) was also pointed out[63,72]. An important discrepancy between these studies can however be observed, probably related to the different methods used to shape the modified separator.
Hong et al. have highlighted a coordinatively unsaturated and defective Ce-MOF-808 (a microporous Ce(IV) trimesate, composed of Ce-oxoclusters and trimesic acid, with a small tetrahedral cage (~5 Å) and a larger adamantane cage (~18 Å) and a high density of structural defects) and CNT composite[77]. The Ce-MOF-808/CNT composite was coated into the separator, enhancing the adsorption and the catalytic conversion of polysulfides, effectively inhibiting the shuttle effect in Li-S batteries and improving the overall electrochemical performance. The battery had a specific capacity of 838 mAh g-1 after 800 cycles at
In addition to defect engineering and Lewis acid-base interaction, functional groups also play an important role. UiO-66(Zr) derivatives bearing different polar groups (ligands) have also been extensively proposed as an alternative to limit the shuttle effect.
Li et al. designed a composited separator by coating UiO-66(Zr)-NH2 with super P (UiO-66(Zr)-NH2 displays a similar structure to UiO-66 with aminoterephtalic acid linker)[74]. The C-N bridged bond was constructed between UiO-66-NH2 and the carbon moiety, providing an efficient electron conduction path for high catalytic efficiency. Moreover, Guo et al. synthesized a series of isostructural solids, UiO-66(Zr)-X MOFs (X: -2OH, -COOH, -NH2), and investigated the differences in the adsorption and conversion of the polysulfides[75]. They showed that UiO-66-NH2 exhibited the highest polysulfide adsorption capacity. The membrane modified by UiO-66-NH2 could effectively inhibit the shuttling effect of polysulfides. Guo et al. also designed a highly ordered graphene/UiO-66(Zr)-NH2 composite. Embedding the MOF particles between the graphene sheets facilitated the capture of the Li2Sx species, which restricted their diffusion and delivered a capacity of 980 mAh g-1 after 100 cycles at 0.1 C[75]. Furthermore, Lin et al. synthesized a sulfonate-rich UiO-66(Zr)-type MOF by post-synthetic oxidative modification. After grafting -SO3Li groups, the UiO-66(SO3Li)4 featured stronger electronegativity, smaller pores and consequently a higher Li+ conductivity than the pristine UiO-66(Zr)[76].
Moreover, two-dimensional (2D) MOFs have also been explored as they might contribute to a specific confinement effect of the polysulfides. Ponnada et al. prepared a modified separator using a conventional GF separator decorated with Fe or Cu MOF nanosheets displaying a similar structure to HKUST-1[82]. High capacity retention was demonstrated, with 985 and 1,237 mAh g-1, for Cu-BTC and Fe-BTC MOF composites, respectively, after 400 cycles at 1 C. Albeit the authors attributed such performance to Cu or Fe atoms coordinated with oxygen atoms on the surface of ultrathin MOF nanosheets, which could limit the polysulfide diffusion due to Lewis acid-base interaction, these composites also contain bipyridine molecules, which can establish a polar interaction with the polysulfides. Furthermore, the nanosheet morphology facilitated the percolation of Li ions.
Conductive MOFs are also particularly interesting since they can be used independently without adding additional electron conductors, showing great advantages in modified separators, even if one could point out these MOFs are in general by far more expensive (ligands), involve the use of toxic organic solvents and thus are much less scalable compared to benchmark MOFs.
Tian et al. proposed Cu2(CuTCPP) TCPP = 5, 10, 15, 20-tetrakis (4-carboxyphenyl porphyrin)-modified separators, prepared by filtration[78]. The Cu-based microporous MOF exhibits a 2D reticulated network built up from porphyrin carboxylate ligands and Cu2+ dimers with open metal sites, which might enhance the affinity towards polysulfides. Besides the chemical interaction, the size of the channels is smaller (1 nm) than the diameter of the long-chain polysulfide intermediates, suggesting that this MOF also plays a physical barrier role, blocking the polysulfides. After 100 cycles at 0.2 C, the discharge capacity remains at
Finally, the conductive 2D MOF Ni3(HITP)2, a microporous nickel hexaiminotriphenylene MOF with uniform ~1.5 nm 1D channels and channel walls rich in polar sites, has also been explored as a functional separator without any carbon since the MOF intrinsic conductivity shall promote polysulfide reactivation, leading to a decrease of the shuttling and acting as a current collector [Figure 6A]. The insulating PP faced limited short-circuiting, allowing the cell to deliver an impressive capacity of 1,139 mAh g-1 after 100 cycles at 0.2 C[64]. Additionally, Chen et al. also prepared Ni3(HITP)2-modified separator [Figure 6B] and have concluded that the microporosity, moderate hydrophilicity, and conductive properties of this MOF improved both the trapping of polysulfides and the kinetics of the electrochemical reaction[79]. However, the system displayed an average performance of 600 mAh g-1 after 200 cycles at 0.5 C. Comparative cyclic voltammetry curves showed a reduced polarization with the MOF-modified separator [Figure 6C], indicating an improved reaction kinetics. The cyclic voltammetry curves of typical Li-S batteries exhibited two distinct cathodic peaks during discharge and one broad anodic peak with a shoulder during charge. The first cathodic peak, observed at ~2.3-2.4 V, represented the solid-to-liquid phase transition, where elemental sulfur (S8) was reduced to higher-order soluble lithium polysulfides (Li2S8 and Li2S6). The second cathodic peak, at ~2.0-2.1 V, corresponded to the liquid-to-solid phase transition, where soluble lithium polysulfides (Li2S6 and Li2S4) were further reduced to insoluble lower-order species such as Li2S2 and Li2S. During the charging process, a broad anodic peak at ~2.3-2.4 V signified the oxidation of Li2S2 and Li2S back to elemental sulfur. Notably, the battery with a modified separator demonstrated a lower oxidation potential and a higher reduction potential than the PP separator. This behavior highlighted the ability of Ni3(HITP)2 to reduce polarization and facilitate the kinetic processes in Li-S batteries, owing to its excellent conductivity and catalytic properties.
Figure 6. (A) Schematic illustration of the interface-induced growth of the conductive Ni3(HITP)2 modified separator for the application in Li-S batteries. 2D layered structure gives Ni3(HITP)2 uniform 1D pore channels, and the Ni3(HITP)2 membrane (black color) grown directly on the surface of the commercial polypropylene separator can be used as a barrier for the suppression of the polysulfide shuttling. Reproduced with permission[64]. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) The illustration of the preparation process of the Ni3(HITP)2-modified separator by filtration and its assembly into the Li-S battery. © 2019, American Chemical Society. (C) Cyclic voltammetry scans of Li-S batteries with PP and Ni3(HITP)2-modified separators at a scan rate of
Some research works have also highlighted the interest in MOF based on bimetallic clusters. Razaq et al. synthesized Fe-doped ZIF-8(Zn), a microporous bimetallic MOF derived from the parent structure of ZIF-8, primarily constructed from zinc ions coordinated with 2-methylimidazole ligands and partially substituted by Fe ions, by a one-step solution direct synthesis method[71]. Compared with the parent ZIF-8, the Fe-doped ZIF-8 exhibited a higher catalytic activity, beneficial for promoting the transformation of polysulfides. The Li-S battery with Fe-ZIF-8/PP as the separator exhibited high cycling stability and capacity retention (409 mAh g-1, 1,000 cycles, 0.5 C). Wang et al. have also proposed a Zn-Co bimetallic MOF
Although numerous examples of separators modified with MOFs and carbons can be found in literature, examples of separators combining MOFs and inorganic moieties are scarce. Yang et al. reported a convenient solvothermal method to convert hierarchical NiCo layered double hydroxide (LDH) into LDH-decorated metal-organic framework (NiCo-MOF/LDH) nanorods, as shown in Figure 7[83]. The composite was then cast on a polyethylene separator and exhibited a good specific capacity of 950 mAh g-1 after 200 cycles at 1 C. Albeit the characterization of the atomic organization of the composite remains unclear, this study proposed an unusual combination of inorganic matter with hybrid materials to improve the polysulfide retention capacity, opening the way to the design of new composites.
Figure 7. The structure of Ni-Co-MOF/LDH is proposed as an interlayer for Li-S batteries. Reproduced with permission[83]. © 2023 Elsevier Ltd. All rights reserved.
MOF/MXenes composites prepared by coating have also been proposed[84]. Ti3C2Tx/Ni-Co MOF heterostructure-modified separators designed by Ren et al. displayed remarkable capacity retention after 350 cycles at 0.1 C, with 1,146 mAh g-1[84]. This behavior was attributed to a synergistic adsorption/electrocatalytic effect caused by the polysulfide confinement into the 2D nanostructures and the presence of unsaturated metal sites of the MOF. The polysulfide shuttling is hence limited due to the strong adsorption of the sulfur species into the 2D/2D heterostructure, effectively promoting their conversion.
In conclusion, although several studies about modified separators are available, there is a lack of harmonization in the practices to prepare and characterize MOF-based modified separators. Nevertheless, the comparison between these composites allows us to highlight the most important features:
• The efficiency of the system does not depend exclusively on the MOF features (structure, pore size and shape, defects, unsaturated sites, functional groups), but should include as well their textural properties (such as particle size and morphology);
• The nature and intrinsic properties of the carbon source (Super P, CNTs, graphene, KB) influence the efficiency of the composite separator, although no clear trend can be drawn;
• The preparation method plays an important role, as does the nature of the supporting separator.
MOF-based self-standing films
In addition to the modified separators, where MOFs and MOF/carbon composites (named MOF/C hereafter) are deposited over the conventional separator, MOFs can also be prepared as self-sustained membranes and directly used as separators. The two main types of MOF-based self-standing films are described below: MOF/C composite membranes and MOF/polymer [Mixed-Matrix Membranes (MMMs)]. Besides being directly used as separators, these films can also be used as interlayers (placed between the cathode and the conventional separator). Such composites have additional requirements compared to modified separators since they need to display enhanced mechanical stability; otherwise, the overall performance of the Li-S battery would be compromised. Table 4 gathers the different types of MOF-based composites shaped as self-sustained films, which will be further developed in the sections below.
Characteristics and electrochemical performance of MOF-based self-sustained films
| MOF (wt%) | Composite type | C and/or polymer moiety (wt%) | Film type | Shaping | Thickness (μm) | Casting density (mg cm-2) | S loading density (mg cm-2) | Specific capacity (mAh g-1) | Areal capacity (mAh cm-2) | Cycle number/cycling rate | Ref. |
| HKUST-1 | MOF/C self-sustained film | GO | Functional separator | Filtration | / | / | 0.6 to 0.8 | 855 | 0.51 to 0.68 | 1,500 at 1 C | [85] |
| HKUST-1(Zn) | GO | 0.2 | / | 0.6 to 0.8 | 675 | 0.40 to0.54 | 1,500 at 1 C | [86] | |||
| HKUST-1 | MOF/polymer membrane | PVDF-HFP | Functional separator | Filtration | / | / | 5.8 | 936 | 5.43 | 200 at 0.1 C | [87] |
| UiO-66-SO3Li (60) | MOF/polymer membrane | PVDF (40) | Functional separator | Blade casting | 22 | / | 2.0 | 580 | 1.16 | 500 at 0.5 C | [58] |
| Co-BDC | MOF/polymer membrane | Bacterial cellulose | Functional separator | Layer-by-layer | 25 | 1.26 | 1.5 | 703 | 1.05 | 200 at 0.5 C | [88] |
| ZIF-67 | MOF/C self-sustained film | CNF | Interlayer | In situ liquid phase growth | 9.4 | 0.35 | 2.3 | 569 | 1.31 | 300 at 1 C | [89] |
| ZIF-7 | MOF/C self-sustained film | CNF | Interlayer | Hydrothermal | / | / | 1.5 | 586 | 0.88 | 500 at 1 C | [90] |
| MOF-74(Ni) (65) | MOF/C self-sustained film | CNT (35) | Interlayer | Secondary solvothermal method | 13-14 | 1.06 | 2.0 | 534 | 1.07 | 1,000 at 5 C | [91] |
| Zr-Fc MOF (90) | MOF/C self-sustained film | CNT (10) | Interlayer | Hydrothermal | 7 | / | 4.11 | 443 | 1.82 | 1,500 at 1 C | [92] |
| ZnPTz | MOF/C self-sustained film | f-CNF | Interlayer | Spray-coating | 8 | 2.5 | 3.5 | 1303 | 4.56 | 200 at 0.1 C | [93] |
| MOF-801(Zr) | MOF/C self-sustained film | CC | Interlayer | Solvothermal | / | / | 2.0 | 751 | 1.50 | 500 at 1 C | [94] |
| MOF-801(Zr) (30) | MOF/C self-sustained film | KB (30) | Interlayer | Blade casting | 15.6 | / | 2.5 | 880 | 2.2 | 50 at 0.1 C | [95] |
| UiO-66(Zr) | MOF/BC self-sustained film | BC | Interlayer | In situ growth | / | 0.02 | / | 739 | / | 100 at 0.5 C | [96] |
MOF composites as functional separators
Carbon-based interlayers have been highlighted as an efficient solution to enhance the performance of Li-S systems due to their unique electronic properties[25,26]. These compounds, such as carbon fibers (CNFs), CNTs, or carbon nanosheets such as graphene oxide (GO), are ideal candidates to ensure the electronic conductivity of the membrane and allow the reactivation of the sulfur species captured within the separator. Their interaction with polysulfides relies mainly on physical confinement. However, numerous studies on heteroatom (N, P, and S)-doped carbons have demonstrated that carbon-based interlayers can also establish a chemical interaction with the trapped polysulfides[97].
Hence, to combine both the selective sieving properties of MOFs and the electronic conductivity of carbonaceous materials, MOF/C composite films have been designed and used as functional separators.
Moreover, MMMs are composite materials containing a filler dispersed in a polymeric matrix. Fillers can display different natures and be inorganic or mineral particles (such as carbon black, graphite, zeolites, metal oxides, and clays) or exhibit a hybrid organic/inorganic character (MOFs)[98,99]. Although the use of such composites has been extensively reported in the literature, particularly for gas separation, preparing scalable MOF-based MMMs with high chemical stability and good mechanical properties remains challenging[100-103]. The chemical affinity between the polymer and the MOF particles is crucial for preparing high-quality films with a good dispersion of the particles. Hence, controlling the particle size and/or morphology is extremely important for interacting with the polymer and homogeneous dispersion of the filler. Moreover, surface functionalization of MOF particles before polymer blending can improve compatibility and dispersion. Particle orientation within the polymer matrix is also important, especially for 2D MOFs, where proper alignment can enhance both permeability and selectivity. In addition to particle characteristics, several other parameters are critical to optimizing MMM fabrication. The preparation method - such as the need to avoid drying particles, the order of component addition, and employing techniques such as sonication - significantly influences membrane quality. Likewise, the choice of membrane fabrication process, whether electrospinning, casting, or another method, affects membrane structure and performance. Another key parameter is the MOF particle loading, since the higher the content of MOFs within the composite, the higher the tendency to create intrinsic defects due to particle agglomeration, which leads to voids. Nonetheless, some polymers allow high loadings, such as PVDF (Polyvinylidene difluoride or PolyVinylidene Fluoride)[104].
The biggest advantage of these films regarding functional separator engineering is the possibility of combining the selective sieving properties of the MOF filler and the enhanced mechanical properties of the polymer support. Hence, these films display a much higher mechanical strength and flexibility than the bare MOF/C films, which are quite brittle[87]. These membranes present a double function in the case of Li-S batteries since, besides mitigating the polysulfide shuttle effect, they can also stabilize the Li plating/stripping process at high current densities and prevent Li dendrites growth [Figure 8][87].
Figure 8. Schematic for Li-S batteries with (A) a routine separator; (B) With a MOF@PVDF-HFP separator. Reproduced with permission[87]. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Zhou et al. have engineered a MOF@PVDF-HFP
MOF-based interlayers
Even though modified separators have been widely studied, both by depositing MOF particles and related derived composites onto a separator surface or by shaping MOF-based self-standing films, limited examples of MOF-based interlayers can be found in literature. Since interlayers are individual components of Li-S systems acting as an extension of the cathode, since interlayers are free-standing membranes, placed between the cathode and the separator, their intrinsic properties are quite different from the ones of a separator, in particular in terms of electric conductivity. Routine separators should avoid a short-circuit of the battery, while an interlayer must display electric conductivity to reactivate the redox process of the sieved polysulfides[95,105-107]. Hence, controlling the properties of a modified separator can certainly be more easily attained. In Scheme 2, a timeline resuming the evolution of interlayers and related features is proposed.
The use of interlayers in Li-S batteries was first introduced by Manthiram[25,108]. in 2012. During the evolution of the design of interlayers, carbon, metal oxide, and MOF particles were successively introduced as important components. MWCNTs, graphene, and CNTs are the most common carbon sources used to prepare interlayers. Although MWCNTs offer good electronic conductivity, the prepared interlayer lacks mechanical properties, especially chemical stability and resistance. GO rapidly replaced MWCNTs due to its combination of electronic conductivity and good mechanical properties (due to its 2D nanosheet structure), showing however poor affinity towards polysulfides. To enhance the affinity, graphene-based composites can be decorated with polar groups, such as hydroxyl, or using oxidizing agents to GO or reduced GO (rGO). Beyond the increasing concern about the eco-toxicity of these carbonaceous materials, the polysulfide affinity remained limited and led rapidly to consider other structures for interlayers. Therefore, metal oxides (TiO2[109,110], MnO2[111,112], V2O5[113,114], Al2O3[115,116]) were then considered. Pristine and relatively abundant metal oxides or more complex ones such as clays (Montmorillonite[117]), or perovskites[118] were tested. The high density of active sites and their porous nature depending on the synthesis conditions are attractive features in a view of polysulfide capture and redox reaction catalysis. Nonetheless, in their pristine form, metal oxides show poor mechanical properties and quite reduced electronic conductivity, leading to systematic combination with carbon-based materials. Finally, since 2016, MOFs have been proposed as alternative systems as they gather adequate and tunable porous structures to capture polysulfides, good mechanical properties for membrane shaping and a high active site density. Provided that MOFs are associated with carbon moieties, or if they bear a natural electronic conductivity, the possibility of these materials in Li-S technology appears almost infinite[49].
Hence, promising combinations with MOFs or derived composites as interlayers have been reported in the past few years. Carbon nanofibers (CNFs) have received particular attention, as they can act as a support for MOFs while offering a conductive network. ZIF-67/CNF composites, prepared by in situ liquid phase growth of ZIF-67 on electrospun CNF, have been described as an efficient interlayer by Li et al.[89]. The efficiency in polysulfide capture of ZIF-67/CNF interlayers was attributed to the microporous nature of ZIF-67 (windows of 3.4 Å and cages of 1.1 nm) and the binding character of the Co sites within the MOF structure. Additionally, the CNF network ensured an efficient electronic percolation between the MOF particles while providing an additional porosity for the PS. After 300 cycles at 1 C, the discharge capacity of the batteries containing this interlayer remained at 569 mAh g-1. Another similar composite, based on ZIF-7/CNF, was reported by Zheng et al.[90]. Similarly to the previous example, the CNF network ensured a high electron percolation whereas the 2D structure of ZIF-7 offered reactive active sites for the polysulfides effective binding and reduction. The composite displayed a specific capacity of 586 mAh g-1 after 500 cycles at 1 C.
In addition to CNFs, CNTs have also been proposed as a conductive support. Li et al. proposed a MOF-74(Ni)/CNT composite, prepared by layer-by-layer seeding technique in solvothermal conditions[91]. MOF-74(Ni) is composed of Ni octahedra chains connected by 2,5-dioxido-1,4-benzenedicarboxylate, with hexagonal honeycomb 1D pores of about 12 Å and available open metal sites upon the desorption of coordinated water molecules. When used as an interlayer, the MOF-74(Ni)/CNT membrane effectively mitigates the polysulfide shuttle effect through its MOF nanopillar array, which acts as a selective sieve. Additionally, the catalytic Ni centers accelerate redox kinetics, promoting rapid polysulfide conversion and enhancing the overall electrochemical performance of the Li-S battery. Simultaneously, this composite also functions as an anode host, where lithium metal is electroplated into the Ni-MOF-74/CNT framework (Li@Ni-MOF-74/CNT). The lithiophilic sites and ordered channels ensure a uniform Li-ion distribution and deposition, effectively mitigating dendrite formation and reinforcing its potential as a protective layer for the lithium anode. Moreover, Wang et al. have prepared a multifunctional interlayer by vacuum filtering CNT and 2D Zr-Fc MOF[92]. Composed of 1,1′-ferrocenedicarboxylic acid [Fc(COOH)2] as ligand and Zr-oxoclusters as subunits, Zr-Fc MOF has positively charged open metal sites that could limit electronegative polysulfides through electrostatic attraction. The one-dimensional CNT hindered the agglomeration of Zr-Fc MOF nanosheets to promote adequate exposure of the active interfaces and contributed to significant long-range conductivity to accelerate electron mobility between MOF nanosheets. Through a combination of techniques, the authors highlighted the electrostatic attraction between positively charged open metal sites in Zr-Fc MOF and electronegative polysulfides and chemical anchoring of Li-O bonding to inhibit polysulfide shuttling and Zr-Fc MOFs provided electrocatalytic effect on polysulfides redox kinetics. The
Chiochan et al. designed a two faces interlayer with conductive functionalized CNF paper (f-CFP) and ZnPTz, or [Zn(H2PO4)2(TzH)2]n, a zinc phosphate composed of octahedral Zn2+ and 1,2,4-triazole linkers[93]. The interlayers exhibited a reversible discharge capacity of 1,303 mAh g-1 after 200 cycles at 0.1 C and showed 0.05% capacity fading per cycle. The Janus interlayer displayed an asymmetric design, with the conductive f-CFP facing the cathode, to reduce the charge transfer resistance and act as a first barrier to limit the soluble lithium polysulfides during the cycling process. On the back side of the interlayer, the crystalline coordination network ZnPTz acted as a second barrier, forming a strong lithium bond interaction for binding the left lithium polysulfides through rich oxygen atoms with lone-pair electrons of phosphate groups on the surface of ZnPTz. Additionally, the ZnPTz particles physically reduced the porosity between the CFP fibers, limiting the polysulfide permeability through physical confinement.
Flexible carbon cloth (CC) has also been considered as an efficient support for MOF growth. Jin et al. have designed a MOF-801(Zr)/CC interlayer through a solvothermal solution growth method[94]. MOF-801(Zr) is a microporous Zr fumarate MOF whose structure displays pores from 5-7 Å and often contains structural defects that act as additional polar sites. The high sieving efficiency of MOF-801(Zr) is attributed to the presence of these sites, together with the presence of micropores and the electronic percolation ensured by the CC support. The composite presented a specific capacity of 751 mAh g-1 after 500 cycles at 1 C. The authors also mentioned the catalytic effect of the Zr6 oxoclusters, which were supposed to enhance the conversion of the long chain polysulfides into short chain ones. Nonetheless, although the catalytic effect is evoked by the authors, such observation is uniquely based on density functional theory (DFT) calculations. Lu et al. have also proposed an interlayer based on MOF-801, mixed with KB as a carbon source and PVDF-HFP. The interlayer, prepared by casting, was included in Li-S cells and displays a discharge capacity of
Besides, MOF/C composites, as new and interesting architectures, started to bloom, such as MOF/cellulose aerogels. Cellulose can display multiple chemical functionalization capabilities, a unique network structure that can further incorporate nanoparticles while having an eco-friendly nature and a worldwide availability[119]. Ma et al. have immobilized UiO-66(Zr) and ZIF-8 particles in bacterial cellulose through a simple in situ growth method, producing a membrane with good mechanical flexibility, a macroscopic character and hierarchical porosity[96]. In particular, the composite aerogel including UiO-66(Zr) particles as a flexible Li-S battery interlayer demonstrates a reversible capacity of 631 mAh g-1 at 0.5 C over 100 cycles, which is attributed to its selectivity for Li+ ions and efficient inhibition of the soluble polysulfide ions. This work seems to be pioneering in the use of biopolymers for Li-S membranes in combination with a green route to synthesize the nanoparticles of MOFs. Thus, cellulose-based composites are of great interest, due to their eco-friendliness and easily scalable shaping conditions. A new strategy to integrate a very high MOF loading into cellulosic papers has been reported by some of us[120]. These MOF/cellulose composites, prepared in green ambient temperature conditions and simply shaped through a filtration method, can lead up to 80% of MOF, while maintaining enhanced mechanical properties, without hampering their sorption properties. This route can easily be applied to all MOFs, opening new avenues for the sustainable shaping of MOF-based membranes for Li-S devices.
CONCLUSION AND OUTLOOK
In this review, we report an overview about MOF-based composites as functional separators and interlayers for Li-S systems, including the different design strategies of membrane architectures, the type of MOF and carbon sources, and the most recent achievements in this field. Also, we highlight how essential it is to differentiate separators and interlayers for a better understanding of the Li-S system and its chemistry.
Although tremendous efforts have been done recently to design efficient systems and mitigate polysulfide shuttling, one can note that the results reported in most studies still lack homogeneity and are also hardly comparable in terms of obtained specific capacities and overall performance of the system. Such discrepancies can be attributed to numerous factors:
• The chemical nature of pristine MOF particles (structure, chemical composition, open metal sites, functional groups) and related physical properties (pore size and shape, particle size and morphology);
• The different physico-chemical properties of the carbon sources (conductivity, porosity, particle size and shape);
• The preparation methods of the MOF/carbon composites and the shaping method of the functional separators;
• The cycling rate and the associated cycle number.
Controlling such a large range of parameters is extremely challenging. Albeit the number of studies concerning MOF-based functional separators has steadily risen in the last five years, fundamental understanding and rational studies of these complex hybrid systems are still required[121,122]. Hence, new perspectives can be proposed to enhance the understanding of these systems:
• Broaden the type of MOF architectures (NB: up to now, mostly benchmark materials such as UiO-66 and related derivatives, ZIF-8, and HKUST-1 have been considered. Exploring less common microporous MOFs with diverse functionalities and structures is crucial to identify optimal pore sizes that can more effectively block polysulfides while allowing rapid lithium-ion transport. Novel designs, such as those incorporating multimetal nodes or heteroatom-functionalized linkers, could provide new opportunities for improved performance.) while working in a rational design of the MOF/carbon composites (not only the carbon type but also the composite synthesis) should be considered, to achieve the perfect balance between the three functions required for efficient functional separators (chemical/physical barrier, conductivity and catalytic activity);
• A comprehensive knowledge about the mechanical properties of the MOF-based composites is required, particularly for the self-sustained films. This is often neglected; nonetheless, such information is essential to understand the long-term performance of the composites when integrated into Li-S cells. Future research should integrate the evaluation of the mechanical properties to shed light on the main parameters that drive the durability of these materials under operational conditions. Similarly, standardized reporting of key parameters such as energy density, the E/S ratio and N/P ratio is critical but often overlooked. These metrics are essential for benchmarking performance and assessing the practical applicability of MOF-based systems. Future studies should prioritize the inclusion of such data to ensure comparability and reproducibility across different research efforts;
• A deeper understanding of the sieving mechanism, in particular through advanced techniques (Solid State Nuclear Magnetic Resonance (ssNMR), X-ray Absorption Spectroscopy (XAS), Raman Spectroscopy, Modeling, and Neutron diffraction), which would allow us to unravel the nature of host-guest interaction at a microscopic scale and therefore establish the crucial structural features that MOF composites should display. Moreover, in situ or operando techniques shall be considered to provide a broader understanding of the chemical interactions and the catalytic processes occurring during polysulfide reduction;
• The scale up of the MOF and related composites should also be taken in account, in particular the synthesis procedure under green and easily scalable conditions. If one considers real life applications and larger devices, the use of less toxic solvents or reactants, cheaper ligands or carbon sources and more sustainable easier preparation methods of composite should be considered in priority. Hardly addressed in the studies until now, the eco-friendly design of MOFs-based functional separators and interlayers should become more systematic. Additionally, for the shaping of the functional separators, the transfer from the lab scale to larger membranes should also be considered, taking into account life cycle analysis (LCA) and technico-economic analysis (TEA) for future industrialization;
• While MOFs have been extensively studied for their potential to enhance the performance of coin-type
Finally, it is worth noting that other porous frameworks have started to blossom as candidates for Li-S devices. This is the case for porous carbon materials[124-126], Polymers of Intrinsic Microporosity (PIMs)[127-129], Porous aromatic frameworks (PAFs)[130-132], or Porous Organic Polymers (POPs)[133]. which include Covalent Organic Frameworks (COFs). These porous materials offer unique properties that address key challenges in Li-S batteries, including mitigating the polysulfide shuttle effect, enhancing sulfur utilization, and improving overall conductivity while also contributing to the suppression of Lithium dendrites. Each material class presents distinct advantages (and limitations), making them valuable options for advancing the performance of Li-S batteries in specific applications. Porous carbon materials provide high electrical conductivity, large pore volumes for sulfur loading and the possibility of being functionalized with heteroatoms (e.g., N, O) for improved polysulfide trapping; PIMs offer lightweight and chemically and thermally stable frameworks with strong polysulfide adsorption capacity; PAFs, interconnected rigid aromatic groups linked by covalent carbon-carbon bonds, display a high specific surface area, associated to an exceptional structural stability and structural functionality; Finally, POPs also appear as an interesting class of materials for such applications. Among POPs, COFs appear as one of the most prominent classes of porous organic compounds. These crystalline frameworks display a high surface area and a versatile structure containing heteroatoms such as B, S, O or N[134]. Albeit COFs lack metal active sites, the heteroatoms within the structure can act as polar sites and interact with lithium polysulfides. Additionally, their 2D character, very common in COFs, enables the easy formation of nanosheets that can be easily integrated into polymeric membranes or even lead to self-standing membranes[135-137]. Thus, the use of COFs as separators or interlayers for Li-S batteries has been recently considered[133,138-140]. Even if these porous frameworks are promising compounds, due to their wide range of structures and pore size and shape, their applications in Li-S devices are still in their infancy. This is certainly related to their synthetic conditions, which are often more complex when compared to MOFs, limiting their ease of scale-up, although recent progress is pointing out[141-144].
DECLARATIONS
Authors’ contributions
Selecting the topic and conceiving the structure of this paper: Lu, W.; Pimenta, V.; Serre, C.
Writing-original draft preparation: Lu, W.; Xu, Y.; Dazon, C.; Macaulay, F.; Pimenta, V.; Serre, C.
Writing-review & editing, supervision: Pimenta, V.; Serre, C.
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This research was supported by the CSC scholarship, grant number 201906880002.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Fan, E.; Li, L.; Wang, Z.; et al. Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects. Chem. Rev. 2020, 120, 7020-63.
2. Bruce, P. G.; Scrosati, B.; Tarascon, J. M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930-46.
3. Shahjalal, M.; Roy, P. K.; Shams, T.; et al. A review on second-life of Li-ion batteries: prospects, challenges, and issues. Energy 2022, 241, 122881.
4. Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on advanced materials for Li-ion batteries. Adv. Mater. 2009, 21, 4593-607.
5. Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energy. Environ. Sci. 2011, 4, 3243.
6. Pope, M. A.; Aksay, I. A. Structural design of cathodes for Li-S batteries. Adv. Energy. Mater. 2015, 5, 1500124.
7. Larcher, D.; Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19-29.
8. Choi, N. S.; Chen, Z.; Freunberger, S. A.; et al. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994-10024.
9. Zhou, G.; Chen, H.; Cui, Y. Formulating energy density for designing practical lithium-sulfur batteries. Nat. Energy. 2022, 7, 312-9.
10. Benveniste, G.; Sánchez, A.; Rallo, H.; Corchero, C.; Amante, B. Comparative life cycle assessment of Li-Sulphur and Li-ion batteries for electric vehicles. Resour. Conserv. Recycl. Adv. 2022, 15, 200086.
11. Benveniste, G.; Rallo, H.; Canals, C. L.; Merino, A.; Amante, B. Comparison of the state of lithium-sulphur and lithium-ion batteries applied to electromobility. J. Environ. Manag. 2018, 226, 1-12.
12. Zhu, K.; Wang, C.; Chi, Z.; et al. How far away are lithium-sulfur batteries from commercialization? Front. Energy. Res. 2019, 7, 123.
13. Huang, Y.; Lin, L.; Zhang, C.; et al. Recent advances and strategies toward polysulfides shuttle inhibition for high-performance Li-S batteries. Adv. Sci. 2022, 9, e2106004.
14. Wang, Z.; Li, Y.; Ji, H.; Zhou, J.; Qian, T.; Yan, C. Unity of opposites between soluble and insoluble lithium polysulfides in lithium-sulfur batteries. Adv. Mater. 2022, 34, e2203699.
15. Hou, L. P.; Li, Z.; Yao, N.; et al. Weakening the solvating power of solvents to encapsulate lithium polysulfides enables long-cycling lithium-sulfur batteries. Adv. Mater. 2022, 34, e2205284.
16. Liu, F.; Lu, W.; Huang, J.; et al. Detangling electrolyte chemical dynamics in lithium sulfur batteries by operando monitoring with optical resonance combs. Nat. Commun. 2023, 14, 7350.
17. Liu, D.; Zhang, C.; Zhou, G.; et al. Catalytic effects in lithium-sulfur batteries: promoted sulfur transformation and reduced shuttle effect. Adv. Sci. 2018, 5, 1700270.
18. Xu, N.; Qian, T.; Liu, X.; Liu, J.; Chen, Y.; Yan, C. Greatly suppressed shuttle effect for improved lithium sulfur battery performance through short chain intermediates. Nano. Lett. 2017, 17, 538-43.
19. Lei, T.; Chen, W.; Lv, W.; et al. Inhibiting polysulfide shuttling with a graphene composite separator for highly robust lithium-sulfur batteries. Joule 2018, 2, 2091-104.
20. Jeong, Y. C.; Kim, J. H.; Nam, S.; Park, C. R.; Yang, S. J. Rational design of nanostructured functional interlayer/separator for advanced Li-S batteries. Adv. Funct. Mater. 2018, 28, 1707411.
21. Xiang, Y.; Li, J.; Lei, J.; et al. Advanced separators for lithium-ion and lithium-sulfur batteries: a review of recent progress. ChemSusChem 2016, 9, 3023-39.
22. Huang, J.; Zhang, Q.; Wei, F. Multi-functional separator/interlayer system for high-stable lithium-sulfur batteries: progress and prospects. Energy. Storage. Mater. 2015, 1, 127-45.
23. Waqas, M.; Niu, Y.; Tang, M.; et al. A decade of development in cathode-facing surface modified separators for high-performance Li-S batteries. Energy. Storage. Mater. 2024, 72, 103682.
24. Chung, S. H.; Manthiram, A. A polyethylene glycol-supported microporous carbon coating as a polysulfide trap for utilizing pure sulfur cathodes in lithium-sulfur batteries. Adv. Mater. 2014, 26, 7352-7.
25. Su, Y. S.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166.
26. Chen, L.; Yu, H.; Li, W.; Dirican, M.; Liu, Y.; Zhang, X. Interlayer design based on carbon materials for lithium-sulfur batteries: a review. J. Mater. Chem. A. 2020, 8, 10709-35.
27. Li, S.; Zhang, W.; Zheng, J.; Lv, M.; Song, H.; Du, L. Inhibition of polysulfide shuttles in Li-S batteries: modified separators and solid-state electrolytes. Adv. Energy. Mater. 2021, 11, 2000779.
28. Chung, S.; Manthiram, A. Bifunctional separator with a light-weight carbon-coating for dynamically and statically stable lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 5299-306.
29. Balach, J.; Jaumann, T.; Klose, M.; Oswald, S.; Eckert, J.; Giebeler, L. Functional mesoporous carbon-coated separator for long-life, high-energy lithium-sulfur batteries. Adv. Funct. Mater. 2015, 25, 5285-91.
30. Guillerm, V.; Ragon, F.; Dan-Hardi, M.; et al. A series of isoreticular, highly stable, porous zirconium oxide based metal-organic frameworks. Angew. Chem. Int. Ed. 2012, 51, 9267-71.
31. Latroche, M.; Surblé, S.; Serre, C.; et al. Hydrogen storage in the giant-pore metal-organic frameworks MIL-100 and MIL-101. Angew. Chem. Int. Ed. 2006, 118, 8407-11.
32. Serre, C.; Férey, G. Hybrid open frameworks. 8. Hydrothermal synthesis, crystal structure, and thermal behavior of the first three-dimensional titanium(IV) diphosphonate with an open structure: Ti3O2(H2O)2(O3P-(CH2)-PO3)2·(H2O)2, or MIL-22. Inorg. Chem. 1999, 38, 5370-3.
33. Gagnon, K. J.; Perry, H. P.; Clearfield, A. Conventional and unconventional metal-organic frameworks based on phosphonate ligands: MOFs and UMOFs. Chem. Rev. 2012, 112, 1034-54.
34. Serre, C.; Groves, J. A.; Lightfoot, P.; et al. Synthesis, structure and properties of related microporous N,N′-piperazinebismethylenephosphonates of aluminum and titanium. Chem. Mater. 2006, 18, 1451-7.
35. Fang, R.; Zhao, S.; Sun, Z.; Wang, D. W.; Cheng, H. M.; Li, F. More reliable lithium-sulfur batteries: status, solutions and prospects. Adv. Mater. 2017, 29, 1606823.
36. Manthiram, A.; Fu, Y.; Chung, S. H.; Zu, C.; Su, Y. S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751-87.
37. Yang, H.; Wang, X. Secondary-component incorporated hollow MOFs and derivatives for catalytic and energy-related applications. Adv. Mater. 2019, 31, e1800743.
38. Xu, Y.; Chen, Z.; Wang, J.; et al. Design of quasi-metal-organic frameworks for solid polymer electrolytes enabling an ultra-stable interface with Li metal anode. Angew. Chem. Int. Ed. 2025, 64, e202416170.
39. Zhou, C.; Li, Z.; Xu, X.; Mai, L. Metal-organic frameworks enable broad strategies for lithium-sulfur batteries. Natl. Sci. Rev. 2021, 8, nwab055.
40. Zhu, D.; Long, T.; Xu, B.; et al. Recent advances in interlayer and separator engineering for lithium-sulfur batteries. J. Energy. Chem. 2021, 57, 41-60.
41. Tao, X.; Yang, Z.; Yan, R.; et al. Engineering MOFs-derived nanoarchitectures with efficient polysulfides catalytic sites for advanced Li-S batteries. Adv. Mater. Technol. 2023, 8, 2200238.
42. Zhou, T.; Liang, J.; Ye, S.; Zhang, Q.; Liu, J. Fundamental, application and opportunities of single atom catalysts for Li-S batteries. Energy. Storage. Mater. 2023, 55, 322-55.
43. Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-organic frameworks for lithium-sulfur batteries. J. Mater. Chem. A. 2019, 7, 3469-91.
44. Jiang, G.; Jiang, N.; Zheng, N.; et al. MOF-derived porous Co3O4-NC nanoflake arrays on carbon fiber cloth as stable hosts for dendrite-free Li metal anodes. Energy. Storage. Mater. 2019, 23, 181-9.
45. Li, Z.; Li, C.; Ge, X.; et al. Reduced graphene oxide wrapped MOFs-derived cobalt-doped porous carbon polyhedrons as sulfur immobilizers as cathodes for high performance lithium sulfur batteries. Nano. Energy. 2016, 23, 15-26.
46. Wang, C.; Song, H.; Yu, C.; et al. Iron single-atom catalyst anchored on nitrogen-rich MOF-derived carbon nanocage to accelerate polysulfide redox conversion for lithium sulfur batteries. J. Mater. Chem. A. 2020, 8, 3421-30.
47. Boyd, D. A. Sulfur and its role in modern materials science. Angew. Chem. Int. Ed. 2016, 55, 15486-502.
48. He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy. Storage. Mater. 2019, 20, 55-70.
49. Suriyakumar, S.; Stephan, A. M. Mitigation of polysulfide shuttling by interlayer/permselective separators in lithium-sulfur batteries. ACS. Appl. Energy. Mater. 2020, 3, 8095-129.
50. Horcajada, P.; Chalati, T.; Serre, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172-8.
51. Islamoglu, T.; Idrees, K. B.; Son, F. A.; et al. Are you using the right probe molecules for assessing the textural properties of metal-organic frameworks? J. Mater. Chem. A. 2021, 10, 157-73.
52. Furukawa, H.; Ko, N.; Go, Y. B.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424-8.
53. Zhang, X.; Chen, Z.; Liu, X.; et al. A historical overview of the activation and porosity of metal-organic frameworks. Chem. Soc. Rev. 2020, 49, 7406-27.
54. Kang, X.; He, T.; Zou, R.; et al. Size effect for inhibiting polysulfides shuttle in lithium-sulfur batteries. Small 2024, 20, e2306503.
55. Chang, Z.; Qiao, Y.; Wang, J.; Deng, H.; He, P.; Zhou, H. Fabricating better metal-organic frameworks separators for Li-S batteries: pore sizes effects inspired channel modification strategy. Energy. Storage. Mater. 2020, 25, 164-71.
56. Hong, X. J.; Tan, T. X.; Guo, Y. K.; et al. Confinement of polysulfides within bi-functional metal-organic frameworks for high performance lithium-sulfur batteries. Nanoscale 2018, 10, 2774-80.
57. Ji, Z.; Han, B.; Li, Q.; et al. Anchoring lithium polysulfides via affinitive interactions: electrostatic attraction, hydrogen bonding, or in parallel? J. Phys. Chem. C. 2015, 119, 20495-502.
58. Wang, Z.; Huang, W.; Hua, J.; et al. An anionic-MOF-based bifunctional separator for regulating lithium deposition and suppressing polysulfides shuttle in Li-S batteries. Small. Methods. 2020, 4, 2000082.
59. Wang, Z.; Wang, B.; Yang, Y.; et al. Mixed-metal-organic framework with effective lewis acidic sites for sulfur confinement in high-performance lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2015, 7, 20999-1004.
60. Zheng, J.; Tian, J.; Wu, D.; et al. Lewis acid-base interactions between polysulfides and metal organic framework in lithium sulfur batteries. Nano. Lett. 2014, 14, 2345-52.
61. He, S.; Yang, J.; Liu, S.; Wang, X.; Qiu, J. A universal MOF-confined strategy to synthesize atomically dispersed metal electrocatalysts toward fast redox conversion in lithium-sulfur batteries. Adv. Funct. Mater. 2024, 34, 2314133.
62. Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon materials for lithium sulfur batteries-ten critical questions. Chemistry 2016, 22, 7324-51.
63. Han, J.; Gao, S.; Wang, R.; et al. Investigation of the mechanism of metal-organic frameworks preventing polysulfide shuttling from the perspective of composition and structure. J. Mater. Chem. A. 2020, 8, 6661-9.
64. Zang, Y.; Pei, F.; Huang, J.; Fu, Z.; Xu, G.; Fang, X. Large-area preparation of crack-free crystalline microporous conductive membrane to upgrade high energy lithium-sulfur batteries. Adv. Energy. Mater. 2018, 8, 1802052.
65. Chung, S. H.; Manthiram, A. High-performance Li-S batteries with an ultra-lightweight MWCNT-coated separator. J. Phys. Chem. Lett. 2014, 5, 1978-83.
66. Yang, H. C.; Xie, Y.; Hou, J.; Cheetham, A. K.; Chen, V.; Darling, S. B. Janus membranes: creating asymmetry for energy efficiency. Adv. Mater. 2018, 30, e1801495.
67. Li, M.; Wan, Y.; Huang, J.; et al. Metal-organic framework-based separators for enhancing Li-S battery stability: mechanism of mitigating polysulfide diffusion. ACS. Energy. Lett. 2017, 2, 2362-7.
68. Yang, H. C.; Hou, J.; Chen, V.; Xu, Z. K. Janus membranes: exploring duality for advanced separation. Angew. Chem. Int. Ed. 2016, 55, 13398-407.
69. Wu, F.; Zhao, S.; Chen, L.; et al. Metal-organic frameworks composites threaded on the CNT knitted separator for suppressing the shuttle effect of lithium sulfur batteries. Energy. Storage. Mater. 2018, 14, 383-91.
70. Ma, B.; Zhang, X.; Deng, X.; et al. Construction of KB@ZIF-8/PP composite separator for lithium-sulfur batteries with enhanced electrochemical performance. Polymers 2021, 13, 4210.
71. Razaq, R.; Din, M. M. U.; Småbråten, D. R.; et al. Synergistic effect of bimetallic MOF modified separator for long cycle life lithium-sulfur batteries. Adv. Energy. Mater. 2024, 14, 2302897.
72. Fan, Y.; Niu, Z.; Zhang, F.; Zhang, R.; Zhao, Y.; Lu, G. Suppressing the shuttle effect in lithium-sulfur batteries by a UiO-66-modified polypropylene separator. ACS. Omega. 2019, 4, 10328-35.
73. Wang, X.; Zhang, X.; Zhao, Y.; et al. Accelerated multi-step sulfur redox reactions in lithium-sulfur batteries enabled by dual defects in metal-organic framework-based catalysts. Angew. Chem. Int. Ed. 2023, 62, e202306901.
74. Li, L.; Tu, H.; Wang, J.; et al. Electrocatalytic MOF-carbon bridged network accelerates Li+-solvents desolvation for high Li+ diffusion toward rapid sulfur redox kinetics. Adv. Funct. Mater. 2023, 33, 2212499.
75. Guo, S.; Xiao, Y.; Wang, J.; et al. Ordered structure of interlayer constructed with metal-organic frameworks improves the performance of lithium-sulfur batteries. Nano. Res. 2021, 14, 4556-62.
76. Lin, S.; Dong, J.; Chen, R.; et al. Lithium sulfonate-rich MOF modified separator enables high performance lithium-sulfur batteries. J. Alloys. Compd. 2023, 965, 171389.
77. Hong, X. J.; Song, C. L.; Yang, Y.; et al. Cerium based metal-organic frameworks as an efficient separator coating catalyzing the conversion of polysulfides for high performance lithium-sulfur batteries. ACS. Nano. 2019, 13, 1923-31.
78. Tian, M.; Pei, F.; Yao, M.; et al. Ultrathin MOF nanosheet assembled highly oriented microporous membrane as an interlayer for lithium-sulfur batteries. Energy. Storage. Mater. 2019, 21, 14-21.
79. Chen, H.; Xiao, Y.; Chen, C.; et al. Conductive MOF-modified separator for mitigating the shuttle effect of lithium-sulfur battery through a filtration method. ACS. Appl. Mater. Interfaces. 2019, 11, 11459-65.
80. Wang, J.; Zhang, X.; Wang, X.; et al. Activation of MOF catalysts with low steric hindrance via undercoordination chemistry for efficient polysulfide conversion in lithium-sulfur battery. Adv. Energy. Mater. 2024, 14, 2402072.
81. Katz, M. J.; Brown, Z. J.; Colón, Y. J.; et al. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449-51.
82. Ponnada, S.; Mansoor, M.; Aslfattahi, N.; et al. Sustainable metal-organic framework co-engineered glass fiber separators for safer and longer cycle life of Li-S batteries. J. Alloys. Compd. 2023, 941, 168962.
83. Yang, Y.; Ma, S.; Xia, M.; et al. Elaborately converting hierarchical NiCo-LDH to rod-like LDH-decorated MOF as interlayer for high-performance lithium-sulfur battery. Mater. Today. Phys. 2023, 35, 101112.
84. Ren, Y.; Zhai, Q.; Wang, B.; et al. Synergistic adsorption-electrocatalysis of 2D/2D heterostructure toward high performance Li-S batteries. Chem. Eng. J. 2022, 439, 135535.
85. Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal-organic framework-based separator for lithium-sulfur batteries. Nat. Energy. 2016, 1, BFnenergy201694.
86. Bai, S.; Zhu, K.; Wu, S.; et al. A long-life lithium-sulphur battery by integrating zinc-organic framework based separator. J. Mater. Chem. A. 2016, 4, 16812-7.
87. He, Y.; Chang, Z.; Wu, S.; et al. Simultaneously inhibiting lithium dendrites growth and polysulfides shuttle by a flexible MOF-based membrane in Li-S batteries. Adv. Energy. Mater. 2018, 8, 1802130.
88. Li, Y.; Lin, S.; Wang, D.; et al. Single atom array mimic on ultrathin MOF nanosheets boosts the safety and life of lithium-sulfur batteries. Adv. Mater. 2020, 32, e1906722.
89. Li, J.; Jiao, C.; Zhu, J.; et al. Hybrid co-based MOF nanoboxes/CNFs interlayer as microreactors for polysulfides-trapping in lithium-sulfur batteries. J. Energy. Chem. 2021, 57, 469-76.
90. Zheng, S.; Sun, D.; Wu, L.; Liu, S.; Liu, G. Carbon fiber supported two-dimensional ZIF-7 interlayer for durable lithium-sulfur battery. J. Alloys. Compd. 2021, 870, 159412.
91. Li, L.; Luo, Y.; Wang, Y.; Zhang, Z.; Wu, F.; Li, J. Rational design of a well-aligned metal-organic framework nanopillar array for superior lithium-sulfur batteries. Chem. Eng. J. 2023, 454, 140043.
92. Wang, Y.; Deng, Z.; Huang, J.; et al. 2D Zr-Fc metal-organic frameworks with highly efficient anchoring and catalytic conversion ability towards polysulfides for advanced Li-S battery. Energy. Storage. Mater. 2021, 36, 466-77.
93. Chiochan, P.; Kaewruang, S.; Phattharasupakun, N.; et al. Chemical adsorption and physical confinement of polysulfides with the janus-faced interlayer for high-performance lithium-sulfur batteries. Sci. Rep. 2017, 7, 17703.
94. Jin, G.; Zhang, J.; Dang, B.; Wu, F.; Li, J. Engineering zirconium-based metal-organic framework-801 films on carbon cloth as shuttle-inhibiting interlayers for lithium-sulfur batteries. Front. Chem. Sci. Eng. 2022, 16, 511-22.
95. Lu, W.; Pang, Z.; Lamaire, A.; et al. Unraveling the mechanisms of zirconium metal-organic frameworks-based mixed-matrix membranes preventing polysulfide shuttling. Small. Sci. 2024, 4, 2300339.
96. Ma, X.; Lou, Y.; Chen, X.; Shi, Z.; Xu, Y. Multifunctional flexible composite aerogels constructed through in-situ growth of metal-organic framework nanoparticles on bacterial cellulose. Chem. Eng. J. 2019, 356, 227-35.
97. Yang, J.; Chen, F.; Li, C.; Bai, T.; Long, B.; Zhou, X. A free-standing sulfur-doped microporous carbon interlayer derived from luffa sponge for high performance lithium-sulfur batteries. J. Mater. Chem. A. 2016, 4, 14324-33.
98. Dechnik, J.; Gascon, J.; Doonan, C. J.; Janiak, C.; Sumby, C. J. Mixed-matrix membranes. Angew. Chem. Int. Ed. 2017, 56, 9292-310.
99. Lu, Y.; Zhang, H.; Chan, J. Y.; et al. Homochiral MOF-polymer mixed matrix membranes for efficient separation of chiral molecules. Angew. Chem. Int. Ed. 2019, 131, 17084-91.
100. Cheng, Y.; Ying, Y.; Japip, S.; et al. Advanced porous materials in mixed matrix membranes. Adv. Mater. 2018, 30, e1802401.
101. Goh, S. H.; Lau, H. S.; Yong, W. F. Metal-organic frameworks (MOFs)-based mixed matrix membranes (MMMs) for gas separation: a review on advanced materials in harsh environmental applications. Small 2022, 18, e2107536.
102. Chung, T.; Jiang, L. Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483-507.
103. Khulbe, K. C.; Matsuura, T.; Feng, C. Y.; Ismail, A. F. Recent development on the effect of water/moisture on the performance of zeolite membrane and MMMs containing zeolite for gas separation; review. RSC. Adv. 2016, 6, 42943-61.
104. Denny, M. S. J.; Cohen, S. M. In situ modification of metal-organic frameworks in mixed-matrix membranes. Angew. Chem. Int. Ed. 2015, 54, 9029-32.
105. Sung, S.; Kim, B. H.; Lee, S.; Choi, S.; Yoon, W. Y. Increasing sulfur utilization in lithium-sulfur batteries by a Co-MOF-74@MWCNT interlayer. J. Energy. Chem. 2021, 60, 186-93.
106. Liang, J.; Sun, Z.; Li, F.; Cheng, H. Carbon materials for Li-S batteries: functional evolution and performance improvement. Energy. Storage. Mater. 2016, 2, 76-106.
107. Leng, X.; Zeng, J.; Yang, M.; et al. Bimetallic Ni-Co MOF@PAN modified electrospun separator enhances high-performance lithium-sulfur batteries. J. Energy. Chem. 2023, 82, 484-96.
108. Su, Y. S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium-sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. 2012, 48, 8817-9.
109. Wei, S. Z.; Li, W.; Cha, J. J.; et al. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium-sulphur batteries. Nat. Commun. 2013, 4, 1331.
110. Gao, P.; Xu, S.; Chen, Z.; et al. Flexible and hierarchically structured sulfur composite cathode based on the carbonized textile for high-performance Li-S batteries. ACS. Appl. Mater. Interfaces. 2018, 10, 3938-47.
111. Li, Z.; Zhang, J.; Lou, X. W. Hollow carbon nanofibers filled with MnO2 nanosheets as efficient sulfur hosts for lithium-sulfur batteries. Angew. Chem. Int. Ed. 2015, 127, 13078-82.
112. Liang, X.; Nazar, L. F. In situ reactive assembly of scalable core-shell sulfur-MnO2 composite cathodes. ACS. Nano. 2016, 10, 4192-8.
113. Carter, R.; Oakes, L.; Muralidharan, N.; Cohn, A. P.; Douglas, A.; Pint, C. L. Polysulfide anchoring mechanism revealed by atomic layer deposition of V2O5 and sulfur-filled carbon nanotubes for lithium-sulfur batteries. ACS. Appl. Mater. Interfaces. 2017, 9, 7185-92.
114. Zhang, Y.; Wang, L.; Zhang, A.; et al. Novel V2O5/S composite cathode material for the advanced secondary lithium batteries. Solid. State. Ion. 2010, 181, 835-8.
115. Han, X.; Xu, Y.; Chen, X.; et al. Reactivation of dissolved polysulfides in Li-S batteries based on atomic layer deposition of Al2O3 in nanoporous carbon cloth. Nano. Energy. 2013, 2, 1197-206.
116. Zhang, Z.; Lai, Y.; Zhang, Z.; Zhang, K.; Li, J. Al2O3-coated porous separator for enhanced electrochemical performance of lithium sulfur batteries. Electrochim. Acta. 2014, 129, 55-61.
117. Ahn, W.; Lim, S. N.; Lee, D. U.; Kim, K.; Chen, Z.; Yeon, S. Interaction mechanism between a functionalized protective layer and dissolved polysulfide for extended cycle life of lithium sulfur batteries. J. Mater. Chem. A. 2015, 3, 9461-7.
118. Yim, T.; Han, S. H.; Park, N. H.; et al. Effective polysulfide rejection by dipole-aligned BaTiO3 coated separator in lithium-sulfur batteries. Adv. Funct. Mater. 2016, 26, 7817-23.
119. Zhang, Z.; Fang, Z.; Xiang, Y.; et al. Cellulose-based material in lithium-sulfur batteries: a review. Carbohydr. Polym. 2021, 255, 117469.
120. Tignol, P.; Pimenta, V.; Dupont, A. L.; et al. A Sustainable one-pot preparation method for very high porous solids loading paper membranes. ChemRxiv2023.
121. Batyrgali, N.; Yerkinbekova, Y.; Tolganbek, N.; Kalybekkyzy, S.; Bakenov, Z.; Mentbayeva, A. Recent advances on modification of separator for Li/S batteries. ACS. Appl. Energy. Mater. 2023, 6, 588-604.
122. Ryu, J.; Song, W.; Lee, S.; Choi, S.; Park, S. A game changer: functional nano/micromaterials for smart rechargeable batteries. Adv. Funct. Mater. 2020, 30, 1902499.
123. Pathak, A. D.; Cha, E.; Choi, W. Towards the commercialization of Li-S battery: from lab to industry. Energy. Storage. Mater. 2024, 72, 103711.
124. Zheng, Z.; Guo, H.; Pei, F.; et al. High sulfur loading in hierarchical porous carbon rods constructed by vertically oriented porous graphene-like nanosheets for Li-S batteries. Adv. Funct. Mater. 2016, 26, 8952-9.
125. Xiang, Y.; Lu, L.; Kottapalli, A. G. P.; Pei, Y. Status and perspectives of hierarchical porous carbon materials in terms of high-performance lithium-sulfur batteries. Carbon. Energy. 2022, 4, 346-98.
126. Pei, F.; Lin, L.; Fu, A.; et al. A two-dimensional porous carbon-modified separator for high-energy-density Li-S batteries. Joule 2018, 2, 323-36.
127. Doris, S. E.; Ward, A. L.; Frischmann, P. D.; Li, L.; Helms, B. A. Understanding and controlling the chemical evolution and polysulfide-blocking ability of lithium-sulfur battery membranes cast from polymers of intrinsic microporosity. J. Mater. Chem. A. 2016, 4, 16946-52.
128. Hou, J.; Han, L.; Sun, S.; et al. Single-walled carbon nanotubes film supported lithiated PIM-1 ultrathin selective barrier: a multifunctional layer for polypropylene separator to boost performance of Li-S batteries. Polymer 2023, 281, 126137.
129. Liu, W.; Zhang, K.; Ma, L.; et al. An ion sieving conjugated microporous thermoset ultrathin membrane for high-performance Li-S battery. Energy. Storage. Mater. 2022, 49, 1-10.
130. Zhu, K.; Li, Z.; Yu, M.; et al. Multiple boosting Janus membranes synergized with Li-rich PAF-6 and carbon nanoparticles for high performance lithium-sulfur batteries. J. Mater. Chem. A. 2022, 10, 24106-14.
131. Zhang, Z.; Liu, Y.; Li, Z.; et al. Chemical and physical synergism between PAF-54 and SFPEEKK for effective shuttle effect inhibition of lithium-sulfur battery. Mater. Today. Energy. 2023, 38, 101455.
132. Ghasemiestahbanati, E.; Shehzad, A.; Konstas, K.; et al. Exceptional lithium diffusion through porous aromatic framework (PAF) interlayers delivers high capacity and long-life lithium-sulfur batteries. J. Mater. Chem. A. 2022, 10, 902-11.
133. Cheng, Z.; Pan, H.; Zhong, H.; Xiao, Z.; Li, X.; Wang, R. Porous organic polymers for polysulfide trapping in lithium-sulfur batteries. Adv. Funct. Mater. 2018, 28, 1707597.
134. Geng, K.; He, T.; Liu, R.; et al. Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 2020, 120, 8814-933.
135. Sasmal, H. S.; Aiyappa, H. B.; Bhange, S. N.; et al. Superprotonic conductivity in flexible porous covalent organic framework membranes. Angew. Chem. Int. Ed. 2018, 130, 11060-4.
136. Dey, K.; Pal, M.; Rout, K. C.; et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 2017, 139, 13083-91.
137. Dey, K.; Bhunia, S.; Sasmal, H. S.; Reddy, C. M.; Banerjee, R. Self-assembly-driven nanomechanics in porous covalent organic framework thin films. J. Am. Chem. Soc. 2021, 143, 955-63.
138. Yoo, J.; Cho, S. J.; Jung, G. Y.; et al. COF-net on CNT-net as a molecularly designed, hierarchical porous chemical trap for polysulfides in lithium-sulfur batteries. Nano. Lett. 2016, 16, 3292-300.
139. Wang, J.; Si, L.; Wei, Q.; Hong, X.; Cai, S.; Cai, Y. Covalent organic frameworks as the coating layer of ceramic separator for high-efficiency lithium-sulfur batteries. ACS. Appl. Nano. Mater. 2018, 1, 132-8.
140. Hu, B.; Xu, J.; Fan, Z.; et al. Covalent organic framework based lithium-sulfur batteries: materials, interfaces, and solid-state electrolytes. Adv. Energy. Mater. 2023, 13, 2203540.
141. Koner, K.; Sasmal, H. S.; Shetty, D.; Banerjee, R. Thickness-driven synthesis and applications of covalent organic framework nanosheets. Angew. Chem. Int. Ed. 2024, 136, e202406418.
142. Sasmal, H. S.; Kumar, M. A.; Majumder, P.; Banerjee, R. Landscaping covalent organic framework nanomorphologies. J. Am. Chem. Soc. 2022, 144, 11482-98.
143. Kandambeth, S.; Dey, K.; Banerjee, R. Covalent organic frameworks: chemistry beyond the structure. J. Am. Chem. Soc. 2019, 141, 1807-22.
Cite This Article
How to Cite
Lu, W.; Xu, Y.; Dazon, C.; Macaulay, F.; Pimenta, V.; Serre, C. MOF and related composites as selective functional separators and interlayers for Li-S batteries. Energy Mater. 2025, 5, 500075. http://dx.doi.org/10.20517/energymater.2024.260
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















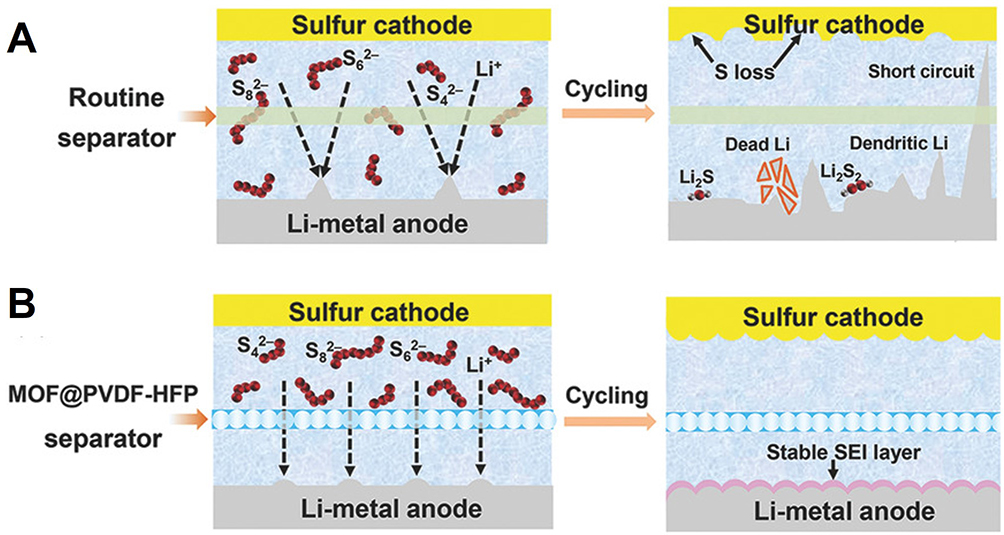














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].