MOF-derived rose-like carbon-coated Ni-Co phosphide with phosphorus vacancies to enhance hydroxide-ion storage in hybrid supercapacitors
Abstract
The low structural stability and sluggish charge-transfer kinetics of transition metal phosphides (TMPs) hinder their application in hybrid supercapacitors. The realization of advanced OH- storage critically depends on the delicate TMP designs, particularly their chemical composition and structure. Herein, a synergistic engineering approach based on metal-organic framework (MOF)-derived C-coated bimetallic phosphides and P vacancies (Pv) was proposed. Using a Ni-Co-based MOF, a one-step high-temperature carbonization and phosphidation method was employed as the precursor to prepare a rose-like Ni1-xCoxP composite (Ni1-xCoₓP@NC), comprising a N-doped carbon (NC) coating and Pv. Physical characterization and theoretical calculations indicated that the open structure with porous Ni1-xCoxP@NC nanosheets originating from high-temperature pyrolysis of Ni-Co-based MOF provides abundant redox-active sites, and the NC layer offers excellent mechanical support for persistent
Keywords
INTRODUCTION
Supercapacitors are a rapidly advancing class of energy storage and conversion devices owing to their present superior power density and longer life than those of secondary batteries[1]. The current commercial double-layer supercapacitors exhibit energy densities of < 10 Wh kg-1, and the development of high-energy and high-power density electrode materials is crucial for increasing the application value of supercapacitors[2]. Transition metal phosphides (TMPs) have received widespread attention owing to their advantages concerning electrical conductivity and high capacitance[3,4]. Reportedly, Ni- and Co-based phosphides possessing high electrochemical activity are promising materials for fabricating
Among various structural design strategies, defect engineering is an “inherent” approach for effectively modulating the charge distribution to optimize the electrode reaction kinetics[12,13]. For instance, oxygen vacancies (Ov) have been extensively studied in supercapacitors because they can serve as shallow donors to increase the carrier concentration and conductivity of the active materials[14]. Fu et al.[15] prepared yolk-shell-structured MnO2 microspheres comprising Ov, which exhibited a reduced charge-transfer resistance and a superior rate capability. Coincidentally, introduced P vacancies (Pv) can also create active sites to modulate the electronic structure of the TMPs and reduce the OH- reaction barrier to enable electron mobility and enhance the electrochemical activity[16,17]. Li et al.[17] induced Pv in a CoP@FeP2 heterostructure via a biotemplate-derived method. Pv substantially influences the abundance of lone pair electrons in the crystal structure, building massive superhighways in the CoP@FeP2 heterostructure for increasing the electron/OH- transfer rates within the electrode (1,028.8 F g-1 at 5 mV s-1). Zhang et al.[18] employed Ar plasma etch
In addition to introducing “intrinsic” vacancies in materials, an “extrinsic” strategy has been adopted in recent research. By modulating the TMP morphology or introducing functional conductive components, the electrical conductivity and structural stability of TMPs can be considerably enhanced[19]. Carbon
In the past decade, metal-organic frameworks (MOFs) have been demonstrated as ideal sacrificial templates for preparing functional nanomaterials[24]. Our research group previously designed functional electrode materials through the MOF derivative method, including a polyhedral star configuration NiS/Co9S8 heterojunction[25], hollow sphere functional MnO2[26], hollow MnO2 cuboid with active Mn3+ and Ov[27], etc. Compared with the transition metal oxides and sulfides reported above, MOF-derived Ni-Co bimetallic phosphide/carbon composites have outstanding advantages such as higher electronic conductivity, higher specific capacitance, and enhanced rate capability[28]. From the perspective of material design strategies, metal compound/C composites prepared via the MOF-derived method exhibit the following advantages: (1) high preparation efficiencies and mild conditions; (2) large surface areas and tunable pore structures; (3) highly homogeneous metal compound/C composites after pyrolysis by the periodically arranged organic ligands and metal ions[29]. Thus, NiCoP-based composites with functional C coatings derived from MOFs exhibit superior abilities in addressing the abovementioned intrinsic drawbacks to promote the electrochemical reaction kinetics of NiCoP-based electrodes.
Herein, a synergistic engineering approach based on MOF-derived C-coated bimetallic phosphides and Pv engineering is proposed. The Pv-enriched porous N-doped carbon (NC)-coated Ni1-xCoxP (Ni1-xCoxP@NC) comprising a blooming rose-like structure was prepared via one-step high-temperature carbonization and phosphidation of Ni-Co-based MOF precursors. This method ensures the uniform and tight binding of the C layer and metal sites, resulting in a porous TMP composite comprising a rose-like morphology and the inherited MOF matrix. The open structure provides abundant redox-active sites, whereas the C framework offers a high-speed electron-transport pathway, ensuring high capacitance and rate capability of the
EXPERIMENTAL
Materials
All chemicals were analytically graded and used as received without further purification. Ethanol (AR), ethylene glycol (AR), nickel nitrate tetrahydrate [Ni(NO3)2·4H2O] (AR) and cobalt nitrate hexahydrate [Co(NO3)2·6H2O] (AR) were purchased from Tianjin Damao Chemical Reagent Co., Ltd. Nicotinic acid. (AR) was purchased from Shanghai Macklin Biochemical Co., Ltd. Sodium hypophosphite
Synthesis of NiCo-MOF, Ni-MOF and Co-MOF
Typically, 6.4 mmol nicotinic acid, 1 mL deionized water and 0.15 g NaOH were first added in 120 mL mixed solution of ethanol and ethylene glycol (volume ratio = 1:1) under magnetic stirring for 0.5 h. Then 2.66 mmol Ni(NO3)2·4H2O and 1.33 mmol Co(NO3)2·6H2O was added into the above mixture. After vigorous stirring for another 0.5 h, the transparent purple solution was sealed in a 100 mL Teflon-line autoclave and kept at 100 °C for 10 h. The resultant precipitate was collected by centrifugation, washed with ethanol and dried at 60 °C, the product was named as NiCo-MOF. The Ni-MOF, Co-MOF are prepared with the same procedures mentioned above, except the addition amount of metal salt, for the synthesis of Co-MOF and Ni-MOF, the addition amounts of Co(NO3)2·6H2O and Ni(NO3)2·4H2O were adjusted to
Synthesis of phosphorus vacancies Ni1-xCoxP@NC, Ni2P@NC and CoP@NC
The N-doped carbon-coated TMP materials with phosphorus vacancies were obtained by one-step
RESULTS AND DISCUSSION
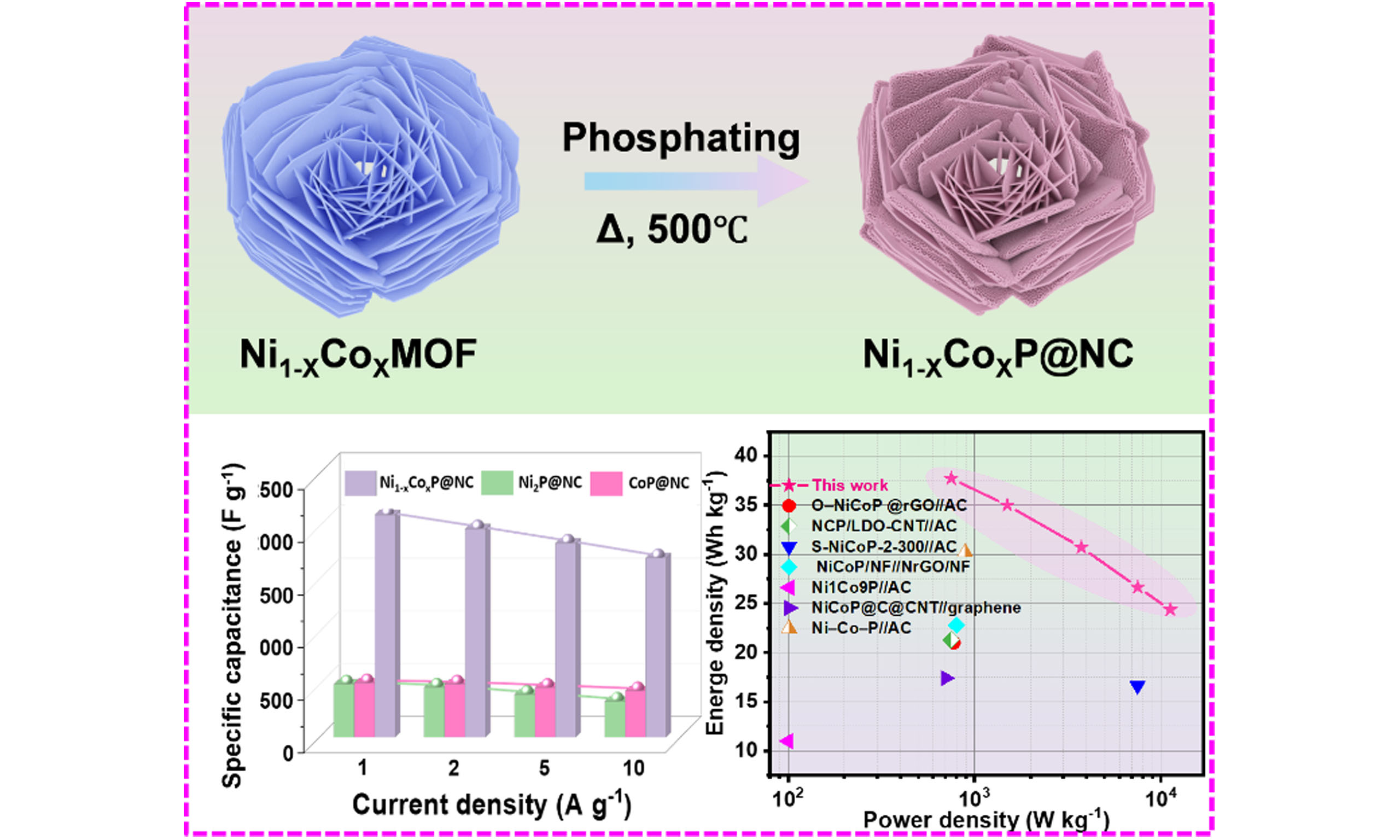
The synthesis procedure of the Ni1-xCoxP@NC composite is illustrated in Figure 1A. First, Co2+ and Ni2+ are subjected to a solvothermal reaction with nicotinic acid in an ethanol/ethylene glycol mixed solution in the presence of trace water and NaOH to obtain NiCo-MOF precursors. Trace water facilitated the dissolution of NaOH in ethanol/ethylene glycol and nicotinic acid deprotonation, thereby promoting crystal nucleation[30]. Scanning electron microscopy (SEM) images [Figure 1B, Supplementary Figure 1A and B] demonstrate that the prepared NiCo-MOF possesses a “blooming rose-like” morphology (diameter≈5 μm), with rose petals comprising 150 nm thick sheets. In contrast, Ni-MOF and Co-MOF prepared using the same method exhibit distinct morphologies [Supplementary Figure 1C-F]. The Ni-MOF presented a uniform “rice-like” morphology (0.3μm × 1.3μm), whereas the Co-MOF demonstrated a “lantern-like” structure (diameter≈10 μm), indicating that the addition of Co2+ during Ni-MOF synthesis induces a transition from its original “rice-like” morphology to a lamellar structure similar to the Co-MOF structure. However, different from the closed “lantern-like” Co-MOF structure, the NiCo-MOF nanoflakes subsequently aggregated into “rose-like” open structures. The NiCo-MOF was then employed as sacrificial templates, and NaH2PO2·H2O was utilized as the P source to directly obtain Ni1-xCoxP@NC via a one-step high-temperature pyrolysis[31]. For comparison, the Ni-MOF and Co-MOF precursors were pyrolyzed under the same conditions to obtain Ni2P@NC and CoP@NC products, respectively. Simultaneously, the PH3 generated during NaH2PO2 decomposition phosphatized the MOF precursor and promoted pore formation in the derivative. SEM images reveal that the Ni1-xCoxP@NC [Figure 1C and Supplementary Figure 2A] and CoP@NC [Supplementary Figure 2B] samples have successfully inherited the morphology of the MOF precursor, indicating the good stability of the structure during the high-temperature treatment. In contrast to the MOF precursor, the “rose petals” of the pyrolyzed Ni1-xCoxP@NC exhibit a uniform porous structure [Figure 1D]. Conversely, Ni2P@NC [Supplementary Figure 2C] displays an irregular granular morphology. During pyrolysis, the carbonization of nicotinic acid yields an NC conductive network for in situ TMP encapsulations. Transmission electron microscopy (TEM) images
Figure 1. Fabrication and microstructure characterization of Ni1-xCoxP@NC composite. (A) The preparation process of rose-like structure Ni1-xCoxP@NC; SEM images of (C) NiCo-MOF (C and D) Ni1-xCoxP@NC; (E and F) TEM images of Ni1-xCoxP@NC; (G) Selected area electron diffraction patterns (up) and enlarged view of the square in Figure 1F (down); (H) Element mapping images of
The X-ray diffraction (XRD) patterns [Supplementary Figure 4A] indicate that the phase structure of
The presence of vacancies was analyzed through electron paramagnetic resonance (EPR) spectroscopy. The broad peak signal (g≈2.0) depicted in Figure 2A indicates the presence of unpaired electrons, corresponding to Pv in the Ni1-xCoxP@NC, CoP@NC, and Ni2P@NC samples[35]. The formation mechanism of Pv is speculated as follows: in the preparation of MOFs, the introduction of Co2+ causes partial damaged original Ni-MOF crystal structure and introduces a large number of defects[36]. Subsequently, during one-step pyrolysis at 500 °C, the redox reactions led to the partly phosphorus loss of the Ni1-xCoxP@NC material and the phosphorus vacancies were induced[33]. The signal intensity of Ni1-xCoxP@NC was higher than that of CoP@NC and Ni2P@NC, indicating a larger number of Pv in Ni1-xCoxP@NC; this is mainly because of the increase in the lattice disorder due to the induction of Co atoms in Ni1-xCoxP@NC. The presence of Pv increases the number of lone electron pairs in Ni1-xCoxP@NC, which would form an electronic transmission channel to boost the electrochemical performance. The nitrogen adsorption-desorption isotherms of
Figure 2. Physical characterization of prepared samples. (A) EPR image of Ni1-xCoxP@NC, Ni2P@NC and CoP@NC composites.
X-ray photoelectron spectroscopy (XPS) analysis was used to investigate the surface chemical state of the samples, and the comprehensive spectrum survey confirmed the coexistence of Ni, Co, P, C, and N in the Ni1-xCoxP@NC, which was consistent with the elemental mapping results [Supplementary Figure 8]. Considering the Ni 2p XPS spectra of the Ni1-xCoxP@NC sample, the peaks at 874.9 and 878.2 eV correspond to 2p1/2, whereas the peak signals at 857.2 and 853.2 eV are attributed to the 2p3/2 of Ni2+
The electrochemical properties of the Ni1-xCoxP@NC composite were evaluated by a three-electrode system in a 6 M KOH solution. The cyclic voltammetry (CV) curve of the Ni1-xCoxP@NC electrode reveals a prominent pair of redox peaks at 0.29 and 0.41 V [Figure 3A] which are derived from its battery-type behavior. The Faradaic redox reactions in the OH- electrolyte can be given as follows[44,45]:
Figure 3. Electrochemical performance of three-electrode system. (A) CV curves of Ni1-xCoxP@NC electrode; (B) Corresponding log (i) vs log (v) plots at specific peaks; (C) Typical diffusion-controlled fraction at 10 mV s-1 of the Ni1-xCoxP@NC electrode (inset is the capacitive and diffusion-controlled charge storage at different scan rates); (D) Bode modulus plots; (E) GCD curves of Ni1-xCoxP@NC electrode; (F) Specific capacitance of Ni1-xCoxP@NC, Ni2P@NC and CoP@NC electrode at different current densities; (G) Comparison of the rate capability with reported NiCo-bimetallic phosphides electrodes; (H) Cycle performance of different electrodes. Ni1-xCoxP@NC: N-doped carbon (NC)-coated Ni1-xCoxP; CV: cyclic voltammetry; GCD: galvanostatic charge/discharge.
The first step is the oxidation process of NiCoP, and the second stage is the further reaction of NiCoP with OH- ions to form NiCoPO. During the redox reaction, several different PO phases would be generated and contribute to extra capacitance. The redox peak potentials of the Ni1-xCoxP@NC electrode are between those of the Ni2P@NC and CoP@NC electrodes, indicating the synergistic effects of Ni and Co on the
Galvanostatic charge/discharge (GCD) tests were performed at the rent rates ranging from 1 to 10 A g-1
To further reveal the intrinsic properties of the as-fabricated samples, the electronic structure and the OH- reaction kinetics were investigated via DFT calculations. The optimization models for Pv-riched Ni1-xCoxP, Ni1-xCoxP without Pv, Ni2P, and CoP are presented in Figure 4A and Supplementary Figure 14. The calculated total density of states (DOS) of Pv-riched Ni1-xCoxP indicates stronger electron delocalization near the Fermi level than that in Ni1-xCoxP without Pv, Ni2P, and CoP, leading to a smaller electronic transition band gap [Figure 4B and Supplementary Figure 15A-C][9,55]. This is primarily attributed to the BIEF induced by the Pv and the synergistic effects of the bimetallic components. The determination of adsorption energy is crucial for understanding the redox reaction kinetics and OH- transfer efficiency between the active material and the electrolyte. Figure 4C shows the OH- adsorption energies for the Pv-rich Ni1-xCoxP, Ni1-xCoxP without Pv, Ni2P, and CoP surfaces are -3.98, -3.93, -3.11, and -3.78 eV, respectively, demonstrating that the synergistic effects between the Ni-Co bimetallic components and Pv can decrease the energy barriers of OH- diffusion and improve the rate capability of the Ni1-xCoxP@NC electrode. The charge distribution of Ni1-xCoxP is shown in Supplementary Figure 16, confirming that the presence of Pv can effectively adjust the charge distribution. The local BIEF is formed around the Pv, which results in an unbalanced charge distribution and generates positively and negatively charged regions, producing the driving force for electron/OH- migration[56,57]. Therefore, the OH- reaction kinetic mechanism of the Pv-rich Ni1-xCoxP@NC electrode has been proposed [Figure 4D]. Prior to charging, owing to its potent adsorption capability, the Pv-rich Ni1-xCoxP@NC material captures abundant OH-, ensuring an ample supply of reacting substances for the electrode reaction. Once charging commences, the BIEF induced by the Pv and the synergistic effects of the NC framework accelerates the electron transfer, boosting the surface reaction of OH-. Such an advanced mechanism maintains the stable operation of electron/OH-, considerably accelerating the kinetic process of the Ni1-xCoxP@NC electrode in the OH--based electrolyte.
Figure 4. Theoretical calculations and physical simulations. (A) Optimized model of and (B) total DOS stimulation of Pv-riched
COMSOL simulations were performed to further explore the physical properties of the Ni1-xCoxP@NC composite. Figure 4E (i) demonstrates that the electric field distribution in the Ni1-xCoxP@NC material is uniform, and strong localized electric fields are not observed. Figure 4E (ii) reveals that a substantial amount of charge is distributed across the surface of the material sheets during the electrode reaction process, indicating the presence of numerous active sites and demonstrating the superior energy-storage capability. Furthermore, the temperature of the Ni1-xCoxP@NC material remained relatively stable throughout the electrochemical reaction, effectively mitigating the high resistance effects typically associated with localized overheating [Figure 4E (iii)]. Finally, a uniform stress distribution was experienced by the Ni1-xCoxP@NC material sheets, indicating that the deformation induced by volume expansion is restricted [Figure 4E (iv)]. The open nanosheet structure can provide good mechanical properties and support each other to reduce the expansion strain caused by the localized stress concentration. The simulation results indicate that the
To explore the practical application potential of Ni1-xCoxP@NC, a pouch-type HSC device was fabricated employing Ni1-xCoxP@NC and AC as the positive and negative electrodes, respectively [Figure 5A]. The charge balance between the positive and negative electrodes in the AC is regulated by adjusting the mass ratio of the constituent materials. The corresponding CV curves are displayed in Figure 5B. As shown in Figure 5C, when the voltage window is 0-1.6 V, considerable oxygen evolution is observed in the anode region of the CV curve. Therefore, the operating voltage of 0-1.5 V was used for the electrochemical analysis of the AC//Ni1-xCoxP@NC HSC. The CV profiles of the AC//Ni1-xCoxP@NC HSC exhibited pronounced redox peaks [Figure 5D], indicating the battery-type mechanism of the device. Despite the increase in the scan rate to 100 mV s-1, the shapes persevered without deterioration, suggesting robust OH-/electron diffusion capabilities inherent to the HSC. The GCD curve of the AC//Ni1-xCoxP@NC HSC shows symmetric shapes, revealing a high Coulombic efficiency [Figure 5E], and the specific capacitances are 121, 112, 98, 85, and 78 F g-1 at 1, 2, 5, 10, and 15 A g-1, respectively, suggesting that the AC//Ni1-xCoxP@NC possesses a superior OH- storage ability [Figure 5F]. The AC//Ni1-xCoxP@NC HSC shows relatively low resistance [Supplementary Figure 17]. The Ragone plot obtained based on the GCD test results reflects the relationship between the power and energy densities of the HSC [Figure 5G]. The assembled
Figure 5. Electrochemical properties of AC//Ni1-xCoxP@NC hybrid supercapacitor. (A) Schematic illustration of AC//Ni1-xCoxP@NC; (B) CV curves of Ni1-xCoxP@NC and AC electrodes in a three-electrode system; (C) CV curves of AC//Ni1-xCoxP@NC in different potential windows at 10 mV s-1; (D) CV curves and (E) GCD curves; (F) Rate capability; (G) Ragone plots of AC//Ni1-xCoxP@NC with reported Ni-Co bimetallic phosphides-based HSC; (H) Cycle performance at 15 A g-1 [inset (left to right): is the external dimensions of AC//Ni1-xCoxP@NC HSC pouch; AC//Ni1-xCoxP@NC HSC pouch keep stable power output under 0°-180° bending and cutting states]. AC: Active carbon; Ni1-xCoxP@NC: N-doped carbon (NC)-coated Ni1-xCoxP; CV: cyclic voltammetry; GCD: galvanostatic charge/discharge; HSC: hybrid supercapacitor.
CONCLUSIONS
In summary, this study presents a strategy based on the MOF-derived method for constructing
DECLARATIONS
Authors’ contributions
Conceived the idea of this study and designed the experiments: Cheng, H.; Cui, Z.; Meng, T.
Performed the material synthesis: Cheng, H.
Contributed to the XRD, SEM, TEM, ICP-OES, N2 adsorption/desorption measurements, EPR and XPS characterization: Cheng, H.; Meng, T.; Cui, Z.; Zheng, W.; Liu, C.; Zeng, Y.; Zheng, J.; Cui, J.; Chen, K.
COMSOL simulations: Zheng, W.
Analyzed the data and wrote the paper: Cheng, H.; Cui, Z.; Meng, T.; Shu, D.
Supervision, funding acquisition: Cheng, H.; Meng, T.; Shu, D.
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Data will be made available upon reasonable request to the corresponding author.
Financial support and sponsorship
The authors wish to acknowledge the following financial supporters of this work: Youth Innovation Talents Project of Guangdong Universities (natural science) (2023KQNCX052); the National Natural Science Foundation of China (Grant No. 22409065); Guangdong Basic and Applied Basic Research Foundation (2022A1515011906); the Postdoctoral Fellowship Program of CPSF (GZC20230868).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Zhang, Y.; Mei, H.; Cao, Y.; et al. Recent advances and challenges of electrode materials for flexible supercapacitors. Coord. Chem. Rev. 2021, 438, 213910.
2. Da, S. L. M.; Cesar, R.; Moreira, C. M.; et al. Reviewing the fundamentals of supercapacitors and the difficulties involving the analysis of the electrochemical findings obtained for porous electrode materials. Energy. Storage. Mater. 2020, 27, 555-90.
3. Acharya, D.; Pathak, I.; Dahal, B.; et al. Immoderate nanoarchitectures of bimetallic MOF derived Ni-Fe-O/NPC on porous carbon nanofibers as freestanding electrode for asymmetric supercapacitors. Carbon 2023, 201, 12-23.
4. Zhu, Y.; Lu, P.; Li, F.; Ding, Y.; Chen, Y. Metal-rich porous copper cobalt phosphide nanoplates as a high-rate and stable battery-type cathode material for battery-supercapacitor hybrid devices. ACS. Appl. Energy. Mater. 2021, 4, 3962-74.
5. Zhang, Y.; Jing, X.; Yan, X.; et al. Rational design of NiMn-based electrode materials for high-performance supercapacitors. Coord. Chem. Rev. 2024, 499, 215494.
6. Patil, S. S.; Patil, P. S. Status review of nickel phosphides for hybrid supercapacitors. Nanoscale 2022, 14, 16731-48.
7. Wen, J.; Xu, B.; Zhou, J. Toward flexible and wearable embroidered supercapacitors from cobalt phosphides-decorated conductive fibers. Nanomicro. Lett. 2019, 11, 89.
8. Nallapureddy, J.; Pallavolu, M. R.; Srinivasa, B. P. S.; Al-asbahi, B. A.; Joo, S. W. Designed construction of hierarchical cobalt sulfide nanonetwork as a high-capacity and binder-free cathode for hybrid supercapacitors. Energy. Fuels. 2023, 37, 17535-44.
9. Wu, Y.; Tao, X.; Qing, Y.; et al. Cr-doped FeNi-P nanoparticles encapsulated into N-doped carbon nanotube as a robust bifunctional catalyst for efficient overall water splitting. Adv. Mater. 2019, 31, e1900178.
10. Zhao, Z.; Miao, Y.; Lu, Q. Electrospun nickel cobalt phosphide/carbon nanofibers as high-performance electrodes for supercapacitors. J. Power. Sources. 2024, 606, 234587.
11. Hussain, N.; Abbas, Z.; Ansari, S. N.; Kedarnath, G.; Mobin, S. M. Phosphorization engineering on a MOF-derived metal phosphide heterostructure (Cu/Cu3P@NC) as an electrode for enhanced supercapacitor performance. Inorg. Chem. 2023, 62, 17083-92.
12. Lu, W.; Yan, L.; Ye, W.; Ning, J.; Zhong, Y.; Hu, Y. Defect engineering of electrode materials towards superior reaction kinetics for high-performance supercapacitors. J. Mater. Chem. A. 2022, 10, 15267-96.
13. Zhang, Y.; Tao, L.; Xie, C.; et al. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, e1905923.
14. Zhang, A.; Gao, R.; Hu, L.; et al. Rich bulk oxygen vacancies-engineered MnO2 with enhanced charge transfer kinetics for supercapacitor. Chem. Eng. J. 2021, 417, 129186.
15. Fu, Y.; Gao, X.; Zha, D.; Zhu, J.; Ouyang, X.; Wang, X. Yolk-shell-structured MnO2 microspheres with oxygen vacancies for high-performance supercapacitors. J. Mater. Chem. A. 2018, 6, 1601-11.
16. Hong, Z.; Zhang, S.; Xia, Y.; et al. Nickel-doped cobalt phosphide with phosphorus-vacancy-abundant as an efficient catalyst for non-aqueous and quasi-solid-state Li-O2 batteries. Mater. Today. Energy. 2024, 43, 101597.
17. Li, K.; Guo, Z.; Sun, Q.; et al. Phosphorus vacancy regulation and interfacial coupling of biotemplate derived CoP@FeP2 heterostructure to boost pseudocapacitive reaction kinetics. Chem. Eng. J. 2023, 454, 140223.
18. Zhang, Q.; Zhang, W.; Ma, X.; et al. Boosting pseudocapacitive energy storage performance via both phosphorus vacancy defect and charge injection technique over the CoP electrode. J. Alloys. Compd. 2021, 864, 158106.
19. Wang, X.; Li, W.; Xu, Y.; et al. NiCoP/C composite with hollow sphere as electrodes for high performance supercapacitors. Electrochim. Acta. 2022, 434, 141313.
20. Tian, W.; Ren, P.; Hou, X.; et al. MnO2 porous carbon composite from cellulose enabling high gravimetric/volumetric performance for supercapacitor. Int. J. Biol. Macromol. 2024, 261, 129977.
21. Zhang, H.; Guo, H.; Zhang, J.; et al. NiCo-MOF directed NiCoP and coconut shell derived porous carbon as high-performance supercapacitor electrodes. J. Energy. Storage. 2022, 54, 105356.
22. Yi, M.; Lu, B.; Zhang, X.; et al. Ionic liquid-assisted synthesis of nickel cobalt phosphide embedded in N, P codoped-carbon with hollow and folded structures for efficient hydrogen evolution reaction and supercapacitor. Appl. Catal. B. Environ. 2021, 283, 119635.
23. Qian, J.; Sun, L.; Shi, X.; et al. Dispersive NiCoP/LDO heterostructure nanosheets scattered by CNTs enabling high-performance electrochemical energy storage. Chem. Eng. J. 2022, 429, 132482.
24. Du, M.; Geng, P.; Feng, W.; Xu, H.; Li, B.; Pang, H. In situ phosphorization for constructing Ni5P2-Ni heterostructure derived from bimetallic MOF for Li-S batteries. Small 2024, 20, e2401587.
25. Cui, Z.; Zheng, W.; Meng, T.; et al. Molecular level heterojunction with sulfur vacancy of stable polyhedral star configuration for boosting hydroxide ion storage. Energy. Storage. Mater. 2024, 71, 103681.
26. Li, Q.; Gao, A.; Meng, T.; et al. Metal-organic framework derived functional MnO2 via an in-situ oxidation strategy for advanced quasi-solid-state supercapacitors. J. Power. Sources. 2023, 560, 232705.
27. Ling, J.; Gao, A.; Huang, Y.; et al. Self-templated and triethanolamine-induced hollow MnO2 nanoboxes with abundant active Mn3+ and oxygen vacancies for high-performance Na-ion pseudocapacitors. Chem. Eng. J. 2023, 452, 139661.
28. Wang, G.; Yi, F.; Zhong, J.; et al. Towards high-performance supercapacitor electrodes via achieving 3D cross-network and favorable surface chemistry. ACS. Appl. Mater. Interfaces. 2022, 14, 34637-48.
29. Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS. Catal. 2016, 6, 5887-903.
30. Li, J.; Yan, D.; Hou, S.; Lu, T.; Yao, Y.; Pan, L. Metal-organic frameworks converted flower-like hybrid with Co3O4 nanoparticles decorated on nitrogen-doped carbon sheets for boosted lithium storage performance. Chem. Eng. J. 2018, 354, 172-81.
31. Zhang, N.; Li, Y.; Xu, J.; et al. High-performance flexible solid-state asymmetric supercapacitors based on bimetallic transition metal phosphide nanocrystals. ACS. Nano. 2019, 13, 10612-21.
32. Lin, Y.; Chen, X.; Tuo, Y.; Pan, Y.; Zhang, J. In-situ doping-induced lattice strain of NiCoP/S nanocrystals for robust wide pH hydrogen evolution electrocatalysis and supercapacitor. J. Energy. Chem. 2022, 70, 27-35.
33. Wang, X.; Liu, X.; Wu, S.; et al. Phosphorus vacancies enriched cobalt phosphide embedded in nitrogen doped carbon matrix enabling seawater splitting at ampere-level current density. Nano. Energy. 2023, 109, 108292.
34. Yang, H.; Xiong, T.; Zhu, Z.; et al. Deciphering the lithium storage chemistry in flexible carbon fiber‐based self‐supportive electrodes. Carbon. Energy. 2022, 4, 820-32.
35. Lin, J.; Yan, Y.; Xu, T.; et al. Rich P vacancies modulate Ni2P/Cu3P interfaced nanosheets for electrocatalytic alkaline water splitting. J. Colloid. Interface. Sci. 2020, 564, 37-42.
36. Jiang, L.; Jiang, L.; Luo, X.; et al. Iron-induced vacancy and electronic regulation of nickle phosphides for ampere-level alkaline water/seawater splitting. Chem. Eng. J. 2024, 502, 157952.
37. Ding, H.; Xu, L.; Wen, C.; et al. Surface and interface engineering of MoNi alloy nanograins bound to Mo-doped NiO nanosheets on 3D graphene foam for high-efficiency water splitting catalysis. Chem. Eng. J. 2022, 440, 135847.
38. Qian, Q.; Zhang, J.; Li, J.; et al. Artificial heterointerfaces achieve delicate reaction kinetics towards hydrogen evolution and hydrazine oxidation catalysis. Angew. Chem. Int. Ed. 2021, 60, 5984-93.
39. Li, Y.; Liu, J.; Chen, C.; Zhang, X.; Chen, J. Preparation of NiCoP hollow quasi-polyhedra and their electrocatalytic properties for hydrogen evolution in alkaline solution. ACS. Appl. Mater. Interfaces. 2017, 9, 5982-91.
40. Zhang, X.; Xue, H.; Sun, J.; et al. Synergy of phosphorus vacancies and build-in electric field into NiCo/NiCoP mott-schottky integrated electrode for enhanced water splitting performance. Chinese. Chem. Lett. 2024, 35, 108519.
41. Sun, R.; Bai, Y.; Bai, Z.; et al. Phosphorus vacancies as effective polysulfide promoter for high‐energy‐density lithium-sulfur batteries. Adv. Energy. Mater. 2022, 12, 2102739.
42. Li, C.; Zhang, H.; Liu, M.; Lang, F.; Pang, J.; Bu, X. Recent progress in metal-organic frameworks (MOFs) for electrocatalysis. Ind. Chem. Mater. 2023, 1, 9-38.
43. Han, Q.; Zhao, X.; Luo, Y.; et al. Synergistic binary Fe-Co nanocluster supported on defective tungsten oxide as efficient oxygen reduction electrocatalyst in zinc-air battery. Adv. Sci. 2022, 9, e2104237.
44. Wang, X.; Jing, C.; Zhang, W.; et al. One-step phosphorization synthesis of CoP@NiCoP nanowire/nanosheet composites hybrid arrays on Ni foam for high-performance supercapacitors. Appl. Surf. Sci. 2020, 532, 147437.
45. Li, X.; Elshahawy, A. M.; Guan, C.; Wang, J. Metal phosphides and phosphates-based electrodes for electrochemical supercapacitors. Small 2017, 13.
46. Xu, W.; Wang, T.; Wang, H.; et al. Free-standing amorphous nanoporous nickel cobalt phosphide prepared by electrochemically delloying process as a high performance energy storage electrode material. Energy. Storage. Mater. 2019, 17, 300-8.
47. Zhang, G.; Hu, J.; Nie, Y.; et al. Integrating flexible ultralight 3D Ni micromesh current collector with NiCo bimetallic hydroxide for smart hybrid supercapacitors. Adv. Funct. Mater. 2021, 31, 2100290.
48. Ren, X.; Li, M.; Qiu, L.; et al. Cationic vacancies and interface engineering on crystalline-amorphous gamma-phase Ni-Co oxyhydroxides achieve ultrahigh mass/areal/volumetric energy density flexible all-solid-state asymmetric supercapacitor. J. Mater. Chem. A. 2023, 11, 5754-65.
49. Jing, C.; Song, X.; Li, K.; et al. Optimizing the rate capability of nickel cobalt phosphide nanowires on graphene oxide by the outer/inter-component synergistic effects. J. Mater. Chem. A. 2020, 8, 1697-708.
50. Zhang, Y.; Sun, L.; Zhang, L.; et al. Highly porous oxygen-doped NiCoP immobilized in reduced graphene oxide for supercapacitive energy storage. Compos. Part. B. Eng. 2020, 182, 107611.
51. Wang, M.; Zhong, J.; Zhu, Z.; et al. Hollow NiCoP nanocubes derived from a Prussian blue analogue self-template for high-performance supercapacitors. J. Alloys. Compd. 2022, 893, 162344.
52. He, S.; Li, Z.; Mi, H.; et al. 3D nickel-cobalt phosphide heterostructure for high-performance solid-state hybrid supercapacitors. J. Power. Sources. 2020, 467, 228324.
53. Zhang, X.; Wu, A.; Wang, X.; Tian, C.; An, R.; Fu, H. Porous NiCoP nanosheets as efficient and stable positive electrodes for advanced asymmetric supercapacitors. J. Mater. Chem. A. 2018, 6, 17905-14.
54. Zhang, X.; Zhang, L.; Xu, G.; Zhao, A.; Zhang, S.; Zhao, T. Template synthesis of structure-controlled 3D hollow nickel-cobalt phosphides microcubes for high-performance supercapacitors. J. Colloid. Interface. Sci. 2020, 561, 23-31.
55. Fu, M.; Chen, W.; Lei, Y.; Yu, H.; Lin, Y.; Terrones, M. Biomimetic construction of ferrite quantum dot/graphene heterostructure for enhancing ion/charge transfer in supercapacitors. Adv. Mater. 2023, 35, e2300940.
56. Pan, L.; Hu, R.; Zhang, Y.; et al. Built-in electric field-driven ultrahigh-rate K-ion storage via heterostructure engineering of dual tellurides integrated with Ti3C2Tx MXene. Nano-Micro. Lett. 2023, 15, 225.
57. Wang, Q.; Yang, H.; Meng, T.; et al. Boosting electron transfer with heterointerface effect for high-performance lithium-ion storage. Energy. Storage. Mater. 2021, 36, 365-75.
58. Li, P.; Han, Y.; Yan, F.; Yan, L.; Huang, H.; Zhou, W. Engineering NiCoP arrays by cross-linked nanowires and nanosheets as advanced materials for hybrid supercapacitors. J. Energy. Storage. 2021, 38, 102503.
59. Gopalakrishnan, A.; Yang, D.; Ince, J. C.; Truong, Y. B.; Yu, A.; Badhulika, S. Facile one-pot synthesis of hollow NiCoP nanospheres via thermal decomposition technique and its free-standing carbon composite for supercapacitor application. J. Energy. Storage. 2019, 25, 100893.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].