Formation of a stable LiF-rich SEI layer on molybdenum-based MXene electrodes for enhanced lithium metal batteries
Abstract
Lithium metal batteries are considered highly promising candidates for the next-generation high-energy storage system. However, the growth of lithium dendrites significantly hinders their advance, particularly under high current densities, due to the formation of unstable solid electrolyte interphase (SEI) layers. In this study, we demonstrate that molybdenum-based MXenes, including Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx, form more stable LiF/Li2CO3 SEI layers during lithium plating, compared to the conventional Cu electrode. Among these, the bimetallic Mo2Ti2C3Tx MXene, with its higher fluorine terminations, produces the most stable LiF-rich SEI layer. The formation of this stable inorganic SEI layer significantly reduces the nucleation overpotential for lithium deposition, promotes uniform Li deposition, and suppresses dendrite growth. Consequently, the Mo2Ti2C3Tx substrate achieved prolonged cycling stability of approximately 544 cycles with coulombic efficiency of ~99.79% at high current density of 3 mA cm-2 and capacity of 1 mAh cm-2. In full cells, the Mo2Ti2C3Tx anode, paired with an NCM622 cathode, maintained capacity retention of 70% over 100 cycles with high cathode loading of 10 mg cm-2. Our approach highlights the potential of Mo-based MXenes to improve the performance of lithium metal batteries, making them promising candidates for the next-generation energy storage system.
Keywords
INTRODUCTION
Lithium metal batteries (LMBs) have garnered significant attention as next-generation power sources due to the high theoretical capacity of lithium metal anodes (3,860 mAh g-1), compared to that of the conventional graphite anodes (372 mAh g-1)[1-3]. However, the practical application of LMBs is currently hindered by their poor cycle life, which results from the uncontrollable growth of lithium dendrites and the collapse of the solid electrolyte interface (SEI) layer on the anode, particularly at high current densities. These issues lead to significant volume change, fast electrolyte consumption, low coulombic efficiency (CE), rapid capacity decay, and ultimately, deteriorated cycling performance[4,5].
The primary roles of the SEI layer are to protect the electrode from unwanted side reactions with lithium metal and to minimize the capacity loss during cycling[6]. The formation of a stable fluorine-rich inorganic SEI layer, such as lithium fluoride (LiF), is imperative for the uniform deposition of Li+ ions at the Li-substrate interface due to its notable characteristics, which include fast ion diffusion, low diffusion energy, and elevated surface energy[7-9].
The F-rich inorganic SEIs can be strategically developed through electrolyte engineering[10,11] and the surface modification of electrodes[12,13]. Despite progress, electrolyte engineering approaches, such as using highly concentrated F-containing salt compositions and fluorinated solvents, face significant challenges for practical implementation. The high salt concentrations required for these electrolytes are difficult to incorporate into commercial battery systems, and replacing conventional solvents with fluorinated ones significantly increases electrolyte viscosity and battery costs[14]. Alternatively, modifying the electrode surface with lithiophilic coatings has been considered the most promising method to form stable F-rich SEI layers[15,16].
MXenes, which are two-dimensional transition metal carbides, nitrides, and carbonitrides, have emerged as potential anode substrates for LMBs, due to the electrochemical activity of their abundant surface termination groups, such as -OH, -O, and -F[17,18]. These negatively charged surface groups make MXene lithiophilic, providing numerous lithium nucleation sites. This characteristic reduces the overpotential during lithium deposition, promotes uniform lithium growth, and suppresses dendrite formation.
To date, titanium (Ti)-based MXenes, such as Ti3C2Tx, have been extensively utilized in LMBs for multiple purposes, including serving as lithiophilic hosts to stabilize the SEI[19], designing 3D MXene-based substrates to mitigate volume changes[20], creating an artificial SEI layer for electrode/electrolyte interface stability[21], and acting as additives to improve the ionic conductivity of solid electrolytes[22]. However, further investigation is urgently required into SEI layers on MXenes that facilitate lithium plating/stripping at higher current densities on commercial Cu substrates. Additionally, designing a suitable MXene anode structure that can maintain a stable SEI layer and enable repeated cycling under high-power operating conditions is critical for next-generation high-energy-density battery applications. Furthermore, despite the development of over 50 types of MXenes since their discovery in 2011, battery research has predominantly concentrated on a few monometallic MXenes, such as Ti3C2Tx, Nb2CTx, and V2CTx, due to the low yield of the synthesized MXenes, and their environmental stability[23].
Herein, we report that three types of Mo-based MXenes - monometallic Mo2CTx and bimetallic Mo2TiC2Tx and Mo2Ti2C3Tx - form more stable inorganic LiF/Li2CO3 SEI layers during lithium plating, compared to the conventional Cu electrode. Notably, the bimetallic Mo2Ti2C3Tx MXene forms the most stable LiF-rich SEI layer, which is attributed to its abundant F-terminations on the surface, which significantly suppresses dendrite growth. Consequently, the Mo2Ti2C3Tx substrate achieves prolonged cycling stability of approximately 544 cycles with CE of ~99.79% at high current density of 3 mA cm-2 and capacity of
EXPERIMENTAL
Materials
Mo2AlC, Mo2TiAlC2, and Mo2Ti2AlC3 MAX-phase powders with average particle sizes of 40 μm were purchased from Jilin 11 Technology (China). Hydrogen fluoride (HF; 48 wt.% in H2O), tetramethylammonium hydroxide (TMAOH; 25 wt.% in H2O), poly(vinylidene fluoride) (PVDF), and Super P carbon were purchased from Sigma-Aldrich. N-methyl-2-pyrrolidone (NMP; 99.5%) was procured from Daejung Chemicals. Commercial NCM622 powders were purchased from MTI-Korea (Republic of Korea). Polypropylene (PP) separator (Celgard 3501) was acquired from MTI-Korea (Republic of Korea). All chemicals were used as received.
Synthesis of the Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes
The Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx MXenes were synthesized by selectively removing Ga from
Preparation of the Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx electrodes
To prepare the MXene electrodes coated on Cu substrates, Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx freeze-dried powders were mixed with poly-vinylidene fluoride and Super P carbon in a mass ratio of 8:1:1 using N-methyl pyrrolidone (NMP) solvent to make a homogeneous slurry. The slurry was coated on the Cu foil using a doctor blade, and dried in a vacuum oven at 80 °C for 8 h to effectively eliminate any residual solvent content. The thickness of Cu foil is 17 µm. The average thickness of the MXene coating layer was approximately 3 μm.
Cell assembly and electrochemical measurements
All electrochemical tests were carried out using 2032-type coin cells that were assembled in an Ar-filled glove box. Half-cell measurements were carried out using a 14 mm diameter MXenes-coated current collector, with Li foil as counter and reference electrode (12 mm in diameter). Celgard 3501 PP membrane was used as a separator in this study. The electrolyte for half-cell was 1 M lithium bis(trifluoromethane sulfonyl) imide (LiTFSI) in 1,3-dioxolane/1,2-dimethoxyethane (DOL/DME;1:1, v/v) with 5 wt.% LiNO3. For full cell measurements, the NCM622 electrode sheets were used as the cathode, and the Li pre-deposited MXene-coated Cu as the anode. The electrolyte for full cells was 1M LiPF6 in ethyl carbonate/dimethyl carbonate (EC/DMC). The electrolyte quantity was fixed at 75 µL. Battery cycling tests were conducted using a WonATech battery cycler. Electrochemical impedance spectroscopy (EIS) was performed using a Bio-Logic VMP3 impedance analyzer at room temperature.
Materials characterization
The X-ray photoelectron spectroscopy (XPS, ESCALAB250 system) analysis was carried out using an
RESULTS AND DISCUSSION
Preparation of the Mo-based MXene anode substrates
Three types of Mo-based MXenes of Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx were synthesized by selectively etching Al or Ga from their corresponding MAX phases (Mo2Ga2C, Mo2TiAlC2, and Mo2Ti2AlC3, respectively), using HF etching solution [Figure 1A]. Detailed synthesis procedures are provided in the Section "EXPERIMENTAL", following previously reported methods. The XRD patterns of the three Mo-based MXenes [Figure 1B] clearly demonstrate the successful etching synthesis, which is evidenced by the shift of the 002 peak toward a lower angle, corresponding to an increase in the interlayer spacing between MXene layers. Additionally, the disappearance of the peak at around 39° suggests the successful etching of the Al or Ga element[26-28]. The TEM images of the Mo2Ti2C3Tx [Figure 1C], Mo2TiC2Tx, and
Figure 1. (A) Schematics of the synthesis of the Mo2Ti2C3Tx,
The surface chemistry and stoichiometry of the Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx films are determined by XPS [Figure 1D and E, Supplementary Figures 4-6]. For the Mo 3d spectra [Figure 1E], the Mo2CTx showed peaks at binding energies (BEs) of 231.98, 230.52, and 233.73 eV, which are attributed to Mo-C, Mo4+, and Mo5+ species, respectively. The Mo2TiC2Tx exhibited contributions from the Mo5+ and Mo6+ at BEs of 230.82 and 235.78 eV, respectively. The Mo2Ti2C3Tx exhibited peaks at 229.57, 230.9, and 234.14 eV, corresponding to the Mo-C and/or Mo-C-Ti/Tx, Mo5+, and Mo6+ species, respectively. The deconvoluted
The fabrication of the Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx electrodes involves preparing a slurry by mixing freeze-dried MXenes powder, PVDF, and super P conducting agent in a weight ratio of 8:1:1 in NMP, then casting the slurry onto a Cu substrate (Details are provided in the Section "EXPERIMENTAL"). The resulting MXene electrodes coated on the Cu substrate exhibited a smooth surface with a coating thickness of approximately 3 µm, as shown in the digital photograph and cross-section scanning electron microscopy (SEM) image [Figure 1G and Supplementary Figure 7]. The uniformity of MXene coating is further confirmed by the energy dispersive spectroscopy (EDS) mapping in [Supplementary Figure 8]. These Mo-based MXene electrodes were then utilized for further electrochemical examinations to investigate their SEI formation, lithium deposition, and dendrite formation behavior in LMBs.
Half-cell battery performance with different electrodes
To investigate the electrochemical performance of the Mo2Ti2C3Tx, Mo2TiC2Tx, Mo2CTx, and bare Cu electrodes, asymmetric half-cells were assembled using lithium metal foil to serve as the counter/reference electrode. PP separator (Celgard 3501) was used, and the electrolyte consisted of 1M LiTFSI in a mixture of DOL and DME with 5 wt.% LiNO3. Galvanostatic lithium metal deposition tests were conducted at a fixed cut-off capacity of 0.5 mAh·cm-2 with an areal current density of 50 uA cm-2 [Figure 2A]. The Mo2Ti2C3Tx||Li electrode exhibited a reduced overpotential of 13 mV, compared to the overpotentials of the Mo2TiC2Tx||Li, Mo2CTx||Li, and bare Cu||Li electrodes of ~16, 21, and 32 mV, respectively, indicating the lowest energy barrier for Li nucleation on the Mo2Ti2C3Tx surface, compared to the other substrates. The overpotential for all the substrates increased with the current density due to ohmic polarization
Figure 2. Half-cell performance test. (A) Nucleation overpotential at current density of 0.05 mA cm-2 with fixed capacity of
In the EIS measurements, the cell with the Mo2Ti2C3Tx electrode exhibited reduced impedance of 11.5 Ω after 200 cycles, compared to the 13, 26, and 44 Ω for the Mo2TiC2Tx, Mo2CTx, and bare Cu electrodes, respectively [Figure 2F]. These experimental results confirm that the Mo-based MXenes substrate facilitates lithium deposition, leading to low overpotential behavior, and superior CE, capacity retention, and cycling performance, compared to the Cu substrate. Among them, the Mo2Ti2C3Tx exhibited the lowest overpotential, the highest Coulombic efficiency, and the most prolonged cycling stability, which surpassed those of all the previously reported studies, including LASS-Cu[30], Cu-Ag[31], Nitrogen-carbon Cu nanorod[32], 3D porous copper[33], ZnO-Cu Zn mesh[34], PNIPAM polymer Cu[35], and Ti3C2Tx Cu[36]
Furthermore, in terms of polymer coatings, a study by Wang et al. demonstrated the use of polyethylene oxide (PEO) for SEI stabilization, achieving stability over 75 cycles at a current density of
When comparing with alloy anodes, the Li-Ge alloy has been used to form a hybrid SEI layer[40], achieving 300 cycles with a CE of 98.5%. Similarly, a lithiophilic alloy film composed of ZnMgTiAl[41] deposited on Cu foil exhibited a CE of 89.5% after 200 cycles at a current density of 2 mA cm-2. These comparisons highlight that the Mo-based MXenes, particularly Mo2Ti2C3Tx, outperformed several other SEI stabilization systems, including polymer coating and alloy anodes. Mo2Ti2C3Tx exhibits long cycling stability of 544 and 298 cycles with high CE, particularly at high current densities of 3 and 5 mA cm-2, underscoring its superior performance.
Morphology of Li deposition on different substrates
To investigate the Li deposition behavior on the MXene electrodes, SEM analysis was performed on the electrode surfaces after 200 cycles at areal capacity of 1 mAh cm-2 and current densities of
Figure 3. Top view SEM images of Li deposited on the Mo2Ti2C3Tx, Mo2TiC2Tx, Mo2CTx, and bare Cu electrodes after 200 cycles at 3 and 5 mA cm-2 with fixed capacity of 1 mAh cm-2. (A-C) The bare Cu, (D-F) Mo2CTx, (G-I) Mo2TiC2Tx, and (J-L) Mo2Ti2C3Tx.
Surprisingly, the bimetallic Mo2TiC2Tx and Mo2Ti2C3Tx MXenes exhibited significant changes in surface morphology. The Mo2TiC2Tx electrode showed much smoother surface morphology, compared to the Cu and Mo2CTx electrodes [Figure 3H and I]. However, small dendrites were still present on the surface at current densities of 3 and 5 mA cm-2, indicating that while the bimetallic Mo2TiC2Tx offers some improvement over the Mo2CTx, it is still insufficient to completely prevent dendrite formation, especially at higher current density of 5 mA cm-2. In contrast, the Mo2Ti2C3Tx electrode demonstrated a more homogeneous and uniform surface morphology [Figure 3K and L]. At a current density of 3 mA cm-2, the Li deposition remained uniform, and free of significant dendritic structures [Figure 3K]. Even at the higher current density of 5 mA cm-2, the Mo2Ti2C3Tx electrode continued to exhibit a smooth and uniform surface [Figure 3L], indicating superior control over Li deposition, compared to the other electrodes. Consequently, the bimetallic Mo2Ti2C3Tx substrate, characterized by the lowest Li nucleation energy barrier, achieved the most uniform lithium deposition, and effectively suppressed dendritic growth, which is attributed to its excellent Coulombic efficiency and extended cycling stability.
Characterization of the solid electrolyte interphase layers
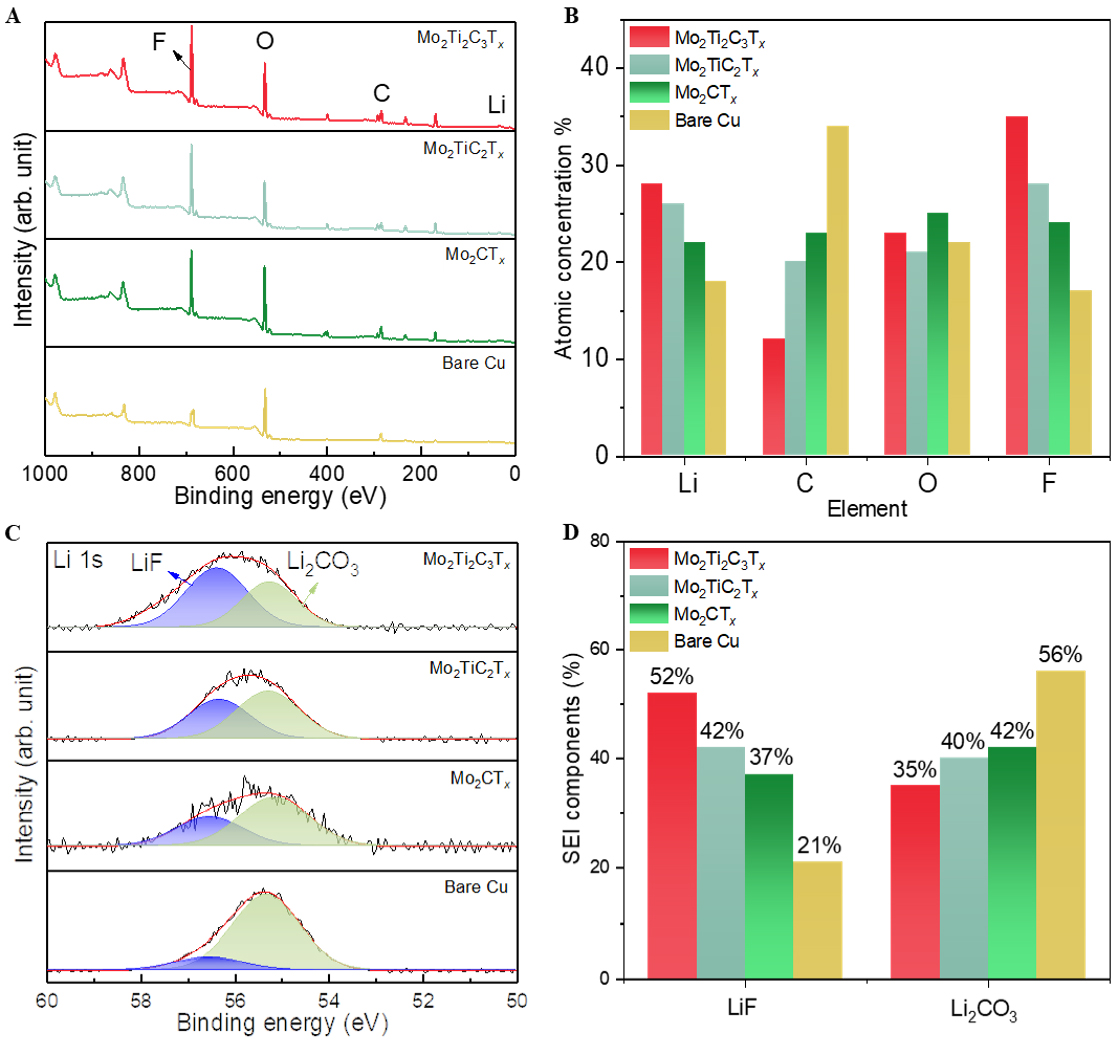
To examine the atomic characteristic of the solid electrolyte interphase (SEI) layers on the Mo2Ti2C3Tx,
Figure 4. XPS analysis of the SEI layer. (A) XPS survey spectra of the Mo2Ti2C3Tx, Mo2TiC2Tx, Mo2CTx, and the bare Cu. (B) Atomic concentration of Li, F, C, and O in the SEI layer. (C) Li 1s XPS spectra. (D) Relative percentage of different components in the SEI layer of the Mo2Ti2C3Tx, Mo2TiC2Tx, Mo2CTx, and bare Cu electrodes.
The increased LiF content in the Mo2Ti2C3Tx, Mo2TiC2Tx, and Mo2CTx, compared to the Cu electrode, can be attributed to the F-terminations on the surface of MXenes, which result from the etching synthesis process, as shown in Figure 1F. The Mo2Ti2C3Tx SEI layer, being the richest in LiF, is likely due to the higher concentration of F-terminations on the surface of the Mo2Ti2C3Tx MXene. Conversely, the Li2CO3 component is relatively low in the Mo2Ti2C3Tx SEI, at 35%, compared to the 40%, 42% and 56% in the
This variation in the chemical composition of the SEI layers significantly influences the electrochemical performance of the Mo2Ti2C3Tx, Mo2TiC2Tx, Mo2CTx, and bare Cu electrodes, as illustrated in Figure 1H. In this study, LiF and Li2CO3 inorganic SEI components were formed on the anode electrodes during charge-discharge cycles. LiF, known for its high ionic conductivity, excellent chemical stability, and robust mechanical strength, is effective at suppressing lithium dendrite growth and enhancing cycling stability[47,48]. Additionally, the inorganic LiF crystals exhibit a wide band gap and a low lithium-ion diffusion barrier, which are crucial for promoting uniform lithium deposition and preventing lithium dendrite formation. Several studies have demonstrated that a LiF-rich SEI layer plays a key role in ensuring homogeneous Li-ion deposition[8]. The high ionic conductivity allows Li+ ions to diffuse through the LiF layer with minimal resistance, while the low diffusion barrier facilitates smooth ion transport across the SEI. This ensures uniform lithium deposition on the electrode surface, effectively minimizing the risk of dendrite formation.
Furthermore, the lithiophilic nature of LiF lowers the energy required for lithium nucleation, reducing nucleation overpotential during the deposition process. This low diffusion energy promotes an even distribution of Li+ ions across the electrode, preventing concentration gradients that typically lead to dendritic growth. By lowering both the diffusion and nucleation energy barriers, LiF ensures a uniform lithium deposition, resulting in a smooth layer instead of sharp dendritic structures. Therefore, the LiF-rich SEI layer plays a crucial role in stabilizing lithium deposition and preventing dendrite formation, significantly enhancing the overall performance of the electrode.
In contrast, Li2CO3, with lower ionic conductivity and stability, is less effective at preventing dendrite formation, and is more prone to degradation, which can limit long-term battery performance[49,50]. Li2CO3 can have both beneficial and detrimental effects on the battery cycling performance depending on its concentration. In small amounts, Li2CO3 assists in the initial SEI formation by providing a protective layer. However, an excess of Li2CO3 is typically detrimental as it increases resistivity, reduces chemical stability, and accelerates SEI degradation. This leads to higher impedance and reduced cycling performance[49].
A LiF-dominant SEI is more favorable for long-term cycling stability due to its lower impedance, ability to promote uniform lithium deposition, and suppression of dendrite formation. In contrast, a Li2CO3-rich SEI is often linked to higher impedance and faster capacity fade, making it less suitable for sustained cycling. Therefore, maintaining a high LiF-to-Li2CO3 ratio in the SEI is essential for enhancing long-term electrochemical performance.
Consequently, from the chemical perspective, the bimetallic Mo2Ti2C3Tx MXenes exhibit a higher density of fluorine terminations on the surface compared to other Mo-based MXenes, such as
Additionally, from an electronic standpoint, the inclusion of Ti in the Mo-based MXene structure enhances its electronic properties by creating additional energy states near the Fermi level, improving electronic conductivity. This is critical for efficient Li-ion transport and deposition in LMBs. Compared to Mo2CTx and Mo2TiC2Tx, the presence of an additional Ti layer in Mo2Ti2C3Tx modifies the electronic structure, increasing both the electron density and the density of states (DOS). This leads to improved charge transfer kinetics during battery cycling[53,54]. Furthermore, the bimetallic Mo2Ti2C3Tx offers a more interconnected lattice structure than other Mo-based MXenes, which enhances Li-ion diffusion pathways and reduces the energy barrier for Li-ion transport[55]. Therefore, the bimetallic Mo-Ti composition in the Mo2Ti2C3Tx plays a crucial role in stabilizing the SEI layer and enhancing battery performance through both chemical and electronic mechanisms.
Full-cell battery performance
The full cell performance of the Mo2Ti2C3Tx, Mo2TiC2Tx, Mo2CTx, and bare Cu anodes, paired with an NCM622 cathode that has a high cathode loading of 10 mg cm-2, was evaluated. The cells, designated as
Figure 5. Full cell performance comparison (A) Cycling performances comparison of the Li/Mo2Ti2C3Tx||NCM622, Li/Mo2TiC2Tx||NCM622, Li/Mo2CTx||NCM622, and Li/Cu||NCM622 at 0.5 C. Voltage profiles of the 2nd and 100th cycle of the (B) Li/Mo2CTx||NCM622, (C) Li/Mo2TiC2Tx||NCM622, and (D) Li/Mo2Ti2C3Tx||NCM622.
CONCLUSION
Three different Mo-based MXenes - Mo2CTx, Mo2TiC2Tx, and Mo2Ti2C3Tx - were synthesized and evaluated for their electrochemical performances as an anode for LMBs. The surface chemistry of these Mo-based MXenes plays a critical role in the formation of the SEI layer. Among them, the bimetallic Mo2Ti2C3Tx exhibited the most stable LiF-rich SEI layer, which is attributed to its abundant F surface terminations. This stabilization of the SEI layer enhances Li-ion transport, enables uniform lithium nucleation and deposition without dendrite growth, and ultimately results in excellent Coulombic efficiency and prolonged cycling performance. Consequently, the Mo2Ti2C3Tx achieved extended cycling stability of 544 cycles with Coulombic efficiency of 99.79% at high current density of 3 mA cm-2 and capacity of 1 mAh cm-2. In full cells, the Mo2Ti2C3Tx anode, paired with an NCM622 cathode, maintained 70% capacity retention over 100 cycles with high cathode loading of 10 mg cm-2. This study underscores the promising potential of Molybdenum-based MXenes as anode materials for the next-generation high-energy-density LMBs.
DECLARATIONS
Author's contribution
Designed the experiment, performed the physical characterization, electrochemical measurements, and data analysis, and wrote the manuscript: Zaman, S.
Performed the physical characterization, and revised the manuscript: Narayanasamy, M.
Designed the schematics, and revised the manuscript: Naqvi, S. M.
Performed experiments, and revised the manuscript: Hassan, T.
Revised the manuscript: Iqbal, A.; Hussain, N.; Cho, S. Y.; Jung, S.
Performed the SEM measurements, and revised the manuscript: Zafar, U.
Assisted with the XPS analysis: Jeong, S.
Conceptualized and coordinated the activities, provided funding for the work, and revised the manuscript: Koo, C. M.
Availability of data and materials
All the data supporting the findings of this study are available within the article and its ESI. Additional data related to this article can be obtained from the corresponding author upon reasonable request.
Financial support and sponsorship
This study was supported by grants from the Basic Science Research Program (2021M3H4A1A03047327 and 2022R1A2C3006227) through the National Research Foundation of Korea, funded by the Ministry of Science, ICT, and Future Planning; the Fundamental R&D Program for Core Technology of Materials and the Industrial Strategic Technology Development Program (20020855), funded by the Ministry of Trade, Industry, and Energy, Republic of Korea; and the National Research Council of Science & Technology (NST), funded by the Korean Government (MSIT) (CRC22031-000). Furthermore, this research was partially supported by the Ministry of Trade, Industry and Energy(MOTIE), Korea Institute for Advancement of Technology(KIAT) through the International Cooperative R&D program (P0028332).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Jiao, S.; Zheng, J.; Li, Q.; et al. Behavior of lithium metal anodes under various capacity utilization and high current density in lithium metal batteries. Joule 2018, 2, 110-24.
2. Li, G.; Liu, Z.; Huang, Q.; et al. Stable metal battery anodes enabled by polyethylenimine sponge hosts by way of electrokinetic effects. Nat. Energy. 2018, 3, 1076-83.
3. Zhao, L.; Ding, B.; Qin, X. Y.; et al. Revisiting the roles of natural graphite in ongoing lithium-ion batteries. Adv. Mater. 2022, 34, e2106704.
4. Kim, J. M.; Engelhard, M. H.; Lu, B.; et al. High current-density-charging lithium metal batteries enabled by double-layer protected lithium metal anode. Adv. Funct. Mater. 2022, 32, 2207172.
5. Wang, T.; Li, Y.; Zhang, J.; et al. Immunizing lithium metal anodes against dendrite growth using protein molecules to achieve high energy batteries. Nat. Commun. 2020, 11, 5429.
6. Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194-206.
7. Cheng, X. B.; Zhang, R.; Zhao, C. Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 2017, 117, 10403-73.
8. Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy. Mater. 2021, 11, 2100046.
9. von Aspern N, Röschenthaler GV, Winter M, Cekic-Laskovic I. Fluorine and lithium: ideal partners for high-performance rechargeable battery electrolytes. Angew. Chem. Int. Ed. 2019, 58, 15978-6000.
10. Alvarado, J.; Schroeder, M. A.; Pollard, T. P.; et al. Bisalt ether electrolytes: a pathway towards lithium metal batteries with Ni-rich cathodes. Energy. Environ. Sci. 2019, 12, 780-94.
11. Weber, R.; Genovese, M.; Louli, A. J.; et al. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy. 2019, 4, 683-9.
12. Umh, H. N.; Park, J.; Yeo, J.; Jung, S.; Nam, I.; Yi, J. Lithium metal anode on a copper dendritic superstructure. Electrochem. Commun. 2019, 99, 27-31.
13. Yan, K.; Lu, Z.; Lee, H. W.; et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy. 2016, 1, 16010.
14. Jiang, G.; Li, F.; Wang, H.; et al. Perspective on high-concentration electrolytes for lithium metal batteries. Small. Struct. 2021, 2, 2000122.
15. Chae, S. U.; Yi, S.; Yoon, J.; et al. Highly defective Ti3CNTx-MXene-based fiber membrane anode for lithium metal batteries. Energy. Storage. Mater. 2022, 52, 76-84.
16. Lee, J. H.; Cho, Y. G.; Gu, D.; Kim, S. J. 2D PdTe2 thin-film-coated current collectors for long-cycling anode-free rechargeable batteries. ACS. Appl. Mater. Interfaces. 2022, 14, 15080-9.
17. Xu, M.; Zhu, Q.; Li, Y.; Gao, Y.; Sun, N.; Xu, B. Atom-dominated relay catalysis of high-entropy MXene promotes cascade polysulfide conversion for lithium-sulfur batteries. Energy. Environ. Sci. 2024, 17, 7735-48.
18. Zhang, D.; Wang, S.; Li, B.; Gong, Y.; Yang, S. Horizontal growth of lithium on parallelly aligned MXene layers towards dendrite-free metallic lithium anodes. Adv. Mater. 2019, 31, e1901820.
19. Ha, S.; Kim, D.; Lim, H. K.; Koo, C. M.; Kim, S. J.; Yun, Y. S. Lithiophilic MXene-guided lithium metal nucleation and growth behavior. Adv. Funct. Mater. 2021, 31, 2101261.
20. Yao, W.; He, S.; Xu, J.; et al. Polypyrrole nanotube sponge host for stable lithium-metal batteries under lean electrolyte conditions. ACS. Sustain. Chem. Eng. 2021, 9, 2543-51.
21. Liu, C.; Yuan, Z.; Chen, K.; et al. MXene-BN-introduced artificial SEI to inhibit dendrite growth of lithium metal batteries. ACS. Appl. Mater. Interfaces. 2023, 15, 56356-64.
22. Narayanasamy, M.; Zaman, S.; Koo, C. M. 2D MXenes for all-solid-state batteries: a comprehensive review. Mater. Today. Energy. 2023, 37, 101405.
23. Anasori, B.; Lukatskaya, M. R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098.
24. Anasori, B.; Xie, Y.; Beidaghi, M.; et al. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS. Nano. 2015, 9, 9507-16.
25. Halim, J.; Kota, S.; Lukatskaya, M. R.; et al. Synthesis and characterization of 2D molybdenum carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118-27.
26. Iqbal, A.; Kwon, J.; Hassan, T.; et al. Environmentally stable and highly crystalline MXenes for multispectral electromagnetic shielding up to millimeter waves. Adv. Funct. Mater. 2024, 2409346.
27. Iqbal, A.; Shahzad, F.; Hantanasirisakul, K.; et al. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride
28. Shahzad, F.; Alhabeb, M.; Hatter, C. B.; et al. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137-40.
29. Halim, J.; Cook, K. M.; Eklund, P.; Rosen, J.; Barsoum, M. W. XPS of cold pressed multilayered and freestanding delaminated 2D thin films of Mo2TiC2Tz and Mo2Ti2C3Tz (MXenes). Appl. Surf. Sci. 2019, 494, 1138-47.
30. Kwon, H. M.; Kim, N. H.; Hong, S. J.; et al. Uniform Li-metal growth on renewable lignin with lithiophilic functional groups derived from wood for high-performance Li-metal batteries. Surf. Interfaces. 2024, 44, 103643.
31. Cui, S.; Zhai, P.; Yang, W.; et al. Large-scale modification of commercial copper foil with lithiophilic metal layer for Li metal battery. Small 2020, 16, e1905620.
32. Yin, D.; Huang, G.; Wang, S.; et al. Free-standing 3D nitrogen-carbon anchored Cu nanorod arrays: in situ derivation from a metal-organic framework and strategy to stabilize lithium metal anodes. J. Mater. Chem. A. 2020, 8, 1425-31.
33. Lin, H.; Zhang, Z.; Wang, Y.; Zhang, X. L.; Tie, Z.; Jin, Z. Template-sacrificed hot fusion construction and nanoseed modification of 3D porous copper nanoscaffold host for stable-cycling lithium metal anodes. Adv. Funct. Mater. 2021, 31, 2102735.
34. Huang, S.; Zhang, W.; Ming, H.; Cao, G.; Fan, L. Z.; Zhang, H. Chemical energy release driven lithiophilic layer on 1 m2 commercial brass mesh toward highly stable lithium metal batteries. Nano. Lett. 2019, 19, 1832-7.
35. Li, N.; Ye, Q.; Zhang, K.; et al. Normalized lithium growth from the nucleation stage for dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. 2019, 131, 18414-9.
36. Yang, D.; Zhao, C.; Lian, R.; et al. Mechanisms of the planar growth of lithium metal enabled by the 2D lattice confinement from a
37. Wang, G.; Chen, C.; Chen, Y.; et al. Self-stabilized and strongly adhesive supramolecular polymer protective layer enables ultrahigh-rate and large-capacity lithium-metal anode. Angew. Chem. Int. Ed. 2020, 59, 2055-60.
38. Fei, G.; Du, Y.; Liu, X.; et al. Suppressing Li dendrite by a guar gum natural polymer film for high-performance lithium metal anodes. J. Appl. Polym. Sci. 2024, 141, e55127.
39. Ma, M.; Guo, X.; Wen, P.; et al. Reactive solid polymer layer: from a single fluoropolymer to divergent fluorinated interface. Angew. Chem. Int. Ed. 2024, 136, e202407304.
40. Chu, F.; Zhou, J.; Liu, J.; Tang, F.; Song, L.; Wu, F. Constructing a fluorinated interface layer enriched with Ge nanoparticles and Li-Ge alloy for stable lithium metal anodes. Nano. Res. 2024, 17, 5148-58.
41. Zhang, L.; Wu, S.; Gao, J.; et al. Multi-component lithiophilic alloy film modified Cu current collector for long-life lithium metal batteries by a novel FCVA Co-deposition system. Small 2024, 20, e2402752.
42. Ding, F.; Xu, W.; Chen, X.; et al. Effects of carbonate solvents and lithium salts on morphology and coulombic efficiency of lithium electrode. J. Electrochem. Soc. 2013, 160, A1894.
43. Hu, X.; Li, Y.; Liu, J.; Wang, Z.; Bai, Y.; Ma, J. Constructing LiF/Li2CO3-rich heterostructured electrode electrolyte interphases by electrolyte additive for 4.5 V well-cycled lithium metal batteries. Sci. Bull. 2023, 68, 1295-305.
44. Peng, J. Y.; Huang, J.; Li, W. J.; et al. A high-performance rechargeable Li-O2 battery with quasi-solid-state electrolyte. Chinese. Phys. B. 2018, 27, 078201.
45. Beheshti, S. H.; Javanbakht, M.; Omidvar, H.; et al. Development, retainment, and assessment of the graphite-electrolyte interphase in Li-ion batteries regarding the functionality of SEI-forming additives. iScience 2022, 25, 103862.
46. Zhang, B.; Ju, Z.; Xie, Q.; et al. Ti3CNTx MXene/rGO scaffolds directing the formation of a robust, layered SEI toward high-rate and long-cycle lithium metal batteries. Energy. Storage. Mater. 2023, 58, 322-31.
47. Li, Z.; Wang, L.; Huang, X.; He, X. Unveiling the mystery of LiF within solid electrolyte interphase in lithium batteries. Small 2024, 20, e2305429.
48. Zheng, J.; Ju, Z.; Zhang, B.; et al. Lithium ion diffusion mechanism on the inorganic components of the solid-electrolyte interphase. J. Mater. Chem. A. 2021, 9, 10251-9.
49. Fan, L.; Zhuang, H. L.; Gao, L.; Lu, Y.; Archer, L. A. Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A. 2017, 5, 3483-92.
50. Ozhabes, Y.; Gunceler, D.; Arias, T. A. Stability and surface diffusion at lithium-electrolyte interphases with connections to dendrite suppression. arXiv 2015, 150405799.
51. Han, B.; Zhang, Z.; Zou, Y.; et al. Poor stability of Li2CO3 in the solid electrolyte interphase of a lithium-metal anode revealed by cryo-electron microscopy. Adv. Mater. 2021, 33, e2100404.
52. Mahne, N.; Renfrew, S. E.; McCloskey, B. D.; Freunberger, S. A. Electrochemical oxidation of lithium carbonate generates singlet oxygen. Angew. Chem. Int. Ed. 2018, 57, 5529-33.
53. Hussain, I.; Amara, U.; Bibi, F.; et al. Mo-based MXenes: synthesis, properties, and applications. Adv. Colloid. Interface. Sci. 2024, 324, 103077.
54. Yang, Y.; Peng, J.; Shi, Z.; Zhang, P.; Arramel, A.; Li, N. Unveiling the key intermediates in electrocatalytic synthesis of urea with CO2 and N2 coupling reactions on double transition-metal MXenes. J. Mater. Chem. A. 2023, 11, 6428-39.
Cite This Article
How to Cite
Zaman, S.; Narayanasamy, M.; Naqvi, S. M.; Hassan, T.; Iqbal, A.; Zafar, U.; Hussain, N.; Jeong, S.; Cho, S. Y.; Jung, S.; Koo, C. M. Formation of a stable LiF-rich SEI layer on molybdenum-based MXene electrodes for enhanced lithium metal batteries. Energy Mater. 2025, 5, 500028. http://dx.doi.org/10.20517/energymater.2024.133
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.