Synergistic design and synthesis of O, N Co-doped hierarchical porous carbon for enhanced supercapacitor performance
Abstract
Carbon-based supercapacitors have emerged as promising energy storage components for renewable energy applications due to the unique combination of various physicochemical characteristics in porous carbon materials (PCMs) that can improve specific capacitance (SC) properties. It is essential to develop a methodical approach that exploits the synergy of these effects in PCMs to achieve superior capacitance performance. In this study, machine learning (ML) provided a clear direction for experiments in the screening of key physicochemical features; SHapley Additive exPlanations analysis on ML indicated that specific surface area and specific doping species had a significant synergistic impact on SC enhancement. Utilizing these insights, an O, N co-doped hierarchical porous carbon (ONPC-900) was synthesized using a synergistic pyrolysis strategy through K2CO3-assisted in-situ thermal exfoliation and nanopore generation. This method leverages the role of carbon nitride (graphite-phase carbon nitride) as an in-situ layer-stacked template and the oxygen (O)-rich properties of the pre-treated lignite, enabling controlled synthesis of graphene-like folded and amorphous hybrid structures engineered for the efficient N and O doping sites and high specific surface area, resulting in an electrode material with enhanced structural adaptability, rapid charge transfer, and diffusion mass transfer capacity. Density functional theory (DFT) calculations further confirmed that pyrrole nitrogen (N-5), carboxyl (-COOH) active sites, and the defect structure formed by pores synergically enhanced the adsorption of electrolyte ions (K+) and electron transfer, improving the SC performance. The optimized ONPC-900 electrode exhibited impressive SC properties of 440 F g-1 (0.5 A g-1), outperforming most coal-based PCMs. This study provides a methodology for designing and synthesizing high SC electrode materials by optimizing the key characteristic parameters of synergism from complex structure-activity relationships through the combination of ML screening, experimental synthesis, and density functional theory validation.
Keywords
INTRODUCTION
Supercapacitors offer promising opportunities for energy storage applications due to their impressive power density, long cycle life, and fast charge/discharge rates[1-3]. However, high-performance supercapacitors are largely dependent on the structural composition and physicochemical characteristics of the electrode materials used. Thus, a critical hurdle in maximizing the potential of supercapacitors is the development design and optimization selection of high-performance electrode materials. Porous carbon materials (PCMs) are carbon-based electrode materials frequently used in supercapacitors to boost their capacitive capabilities owing to their rich pore structure, excellent conductivity, high ion-accessible specific surface area (SSA), and ease of production[4,5]. Previous research has introduced various synthetic strategies for creating porous structures in PCMs, aiming to achieve high SSA and desired pore characteristics[6]. Improving the SSA and optimizing pore structures - including creating sub-nanopore or hierarchical structured pores with micropores, mesopores, and macropores - were considered effective approaches for enhancing the actual specific capacitance (SC) of carbon-based supercapacitors due to the constraints of the adsorption mechanism in electrical double-layer capacitors (EDLCs)[7-9]. Nonetheless, due to the diversity of PCM precursors and the intricate nature of amorphous porous materials, the controlled modulation of morphological microstructure remains a major challenge. Additionally, the inherent synergies of various features such as morphological microstructure, SSA, and pore structure are ignored in the optimization and synthesis of PCMs with high expected performance.
Moreover, the capacitance properties of PCMs can be improved at a higher level by incorporating heteroatoms and adjusting wettability, reactivity, and pseudo-capacitance[10-12]. Lignite, as an O-containing carbon precursor, has gained attention in research because of its wide range of precursor sources,
Herein, we propose an ML-guided key feature screening optimization and interpretable analysis, successfully synthesizing PCMs with superior key features, and a DFT-assisted validation combination method for optimal design of supercapacitor electrode materials with expected performance. To achieve this, SHAP in ML was used to reveal the interaction impacts of characteristics on SC and screen key features that have positive effects on capacitance enhancement. Based on ML interpretability analysis screening of key physicochemical features, we developed a facile in-situ exfoliation and pore-forming engineered synergistic pyrolysis strategy for constructing and synthesis the O, N co-doped hierarchical porous carbon (ONPC) materials. Physicochemical characterization and electrochemical kinetic quantitative analysis were used to evaluate the significant effects of SSA and N, O co-doped effective active species (such as N-5,
EXPERIMENTAL
Materials
A typical raw lignite named low-rank coal was collected from the Inner Mongolia Autonomous Region of China. To eliminate the influence of other minerals in the raw lignite, demineralization was carried out using hydrofluoric acid (HF, approximately 20 wt%) and hydrochloric acid (HCl, about 1 M) until the ash content was reduced to less than 1%, resulting in the production of ash-free coal known as pre-treated lignite. The approximate and ultimate analysis results of both raw lignite and pre-treated lignite are presented in Supplementary Table 1. The reagents used included potassium carbonate (K2CO3), melamine, sodium hydroxide (NaOH), N, N-dimethylformamide, potassium hydroxide (KOH, ≥ 85%) were purchased from Sinopharm Chemical, polyvinylidene difluoride (PVDF, Shanghai Aladdin Reagent Co., ltd.),
Preparation of ONPC materials
ONPC materials were synthesized using the pre-treated lignite and bulk g-C3N4 as carbon precursors and heteroatomic dopants. The bulk g-C3N4 was synthesized via a previously reported method involving thermal condensation of melamine at 550 °C for 2 h, followed by natural cooling[33]. Subsequently, the bulk g-C3N4 was mixed with K2CO3 and pre-treated lignite in a 1:1:1 mass ratio, ball milled thoroughly, and pyrolyzed at different temperatures (700 °C, 800 °C, and 900 °C) under a N2 atmosphere for 3 h. After natural cooling down to room temperature, the reaction product was washed sequentially with an alkali solution and distilled water and finally vacuum dried at 80 °C for 24 h. More specifically, the obtained samples by pyrolysis were ground and placed in 1 M sodium hydroxide solution (mass volume ratio of sample and washing solution: 1 g/10 mL) and washed at 25 °C for 24 h, then centrifuged at 8,000 rpm and purified several times with distilled water until neutral. The final purified sample was obtained by vacuum drying at 80 °C for 24 h after washing. The resulting PCMs were named ONPC-x (x = 700, 800, and 900). For the sake of contrast, the NPC-900 was also obtained at 900 °C pyrolysis by the same procedure and conditions from the pre-treated lignite and g-C3N4 as carbon and nitrogen precursors, respectively, without the addition of K2CO3. In addition, the synthetic procedure of Oxygen-rich porous carbon (OPC)-900 was similar to that of NPC-900 except for the addition of g-C3N4. The synthesized ONPC materials included ONPC-700,
Material characterization
The microstructure and morphology of ONPC samples were investigated using a ZEISS Sigma 300 scanning electron microscope (SEM). Transmission electron microscope (TEM) images and high-resolution TEM (HRTEM) images were acquired on a JEM-F200 (JEOL) TEM coupled with an energy dispersive spectrometer (EDS; JED-2300T). Thermogravimetric curve (TG, NETZSCH, STA449F5) measurement was carried out to study the pyrolysis process of pre-treated lignite, g-C3N4, and the mixture precursor after ball milling, respectively. The test conditions are: under Ar protection atmosphere, the heating rate of
Electrochemical measurement and analysis
Electrochemical evaluation was conducted utilizing a standard three or two electrode configurations, with all measurements analyzed using a CHI760E (Shanghai Chenhua). In the three-electrode cell, the Pt plate and Hg/HgO served as the counter and reference electrodes, respectively, in a 6 M KOH aqueous electrolyte solution. Prior to the electrochemical tests, the working electrodes were prepared. The preparation process involved grinding an active material with a mass fraction of 80% along with carbon black (10%) in an agate mortar. This mixture was then stirred with N-methyl pyrrolidone as the solvent and polyvinylidene fluoride (PVDF, 10%) to create a mixed electrode slurry. The prepared mixture was drip-coated evenly onto a carbon cloth (1 cm × 1 cm) and dried overnight in a vacuum oven at 80 °C. The weight of the active material after drying was 1.28 mg (1 cm × 1 cm). The samples underwent testing through cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS). The electrochemical properties of each PCM were investigated by measuring the CV plots at different scan rates at -1~0 V. GCD was performed in the current density range of 0.5 to 20 A g-1 under the same voltage window. EIS plots were obtained in a frequency range of 0.01 kHz to 100 kHz with an applied amplitude of 5 mV, and fitting analysis was conducted using an equivalent circuit diagram. The gravimetric SC (C, F g-1) of a three/two-electrode device is formulated as follows:
where the I (A), ΔV (V), Δt (s), and m (g) represent discharge current (A), test potential during discharging (V), discharge time (s), and the weight of single electrode active material, respectively.
Two electrodes with identical active mass and electrolyte (6M KOH) were incorporated into a symmetric two-electrode supercapacitor system. The electrochemical properties were measured in the voltage window of 0~1 V. The cycle stability of the device was evaluated using a Landt G340A precision battery test system.
The energy densities (E, Wh kg-1) and power densities (P, W kg-1) were rigorously determined by:
Machine learning-assisted analysis
Data collection and feature engineering
To elucidate the relationship between SC and porous carbon structural characteristics for designing
ML model evaluation and interpretation
Research has demonstrated that Random Forest (RF) modeling is more feasible and efficient than other methods for evaluating feature importance, particularly in high-latitude data. Therefore, the RF model was adopted as the predictive model in this study and trained according to the data collected in the literature. The ML algorithms for the RF models were implemented in a Python programming environment, with the primary ML package being scikit-learn. To begin the analytical process and provide an overview of correlation analysis based on the dataset, all considered feature variables were evaluated using the Pearson correlation coefficient (PCC). This coefficient measures linear correlation between feature variables, primarily to assess collinearity and the relationship between the independent (porous structure) and dependent or target (capacitive performance) variables. The reliability of ML models was assessed using various measurement methods, including the PCC, Mean Absolute Error (MAE), Root Mean Square Error (RMSE), and R-square (R2) between the expected and estimated outputs from the ML model. These parameters can be calculated as follows:
where n represents the number of samples, yi is the real capacitance,
DFT calculations
The heteroatomic doping structure of the electrode material was optimized utilizing DFT calculations with the Vienna Ab initio Simulation Package (VASP)[34]. The calculations employed a plane wave base set with an energy cutoff of 600 eV, a projected-enhanced wave pseudo-potential, and a generalized gradient approximation of the exchange-correlation functional parameterized by Perdew, Burke, and Ernzerhof. The model structure underwent full optimization for ionic and electronic degrees of freedom, with electronic energy convergence criteria set at 10-5 eV and force convergence criteria at 10-2 eV/Å for each atom. The absorbed electrolyte energy (Ea) of the electrolyte ion (K+) on an undoped or doped carbon surface is defined by:
where Etotal represents the total energy after electrolyte ion adsorption, Esurf is the undoped or doped carbon surface energy, and EK+ is the energy of a single K+ in the bulk position.
RESULTS AND DISCUSSION
Considering the advantages of ML, which can reduce experimental and computational costs and accelerate the discovery and optimization of new materials, we chose the influence of multi-physicochemical characteristic parameters on the SC performance of supercapacitor electrode materials as the research point. By gathering existing literature data and building a ML model, combined with comprehensive interpretability analysis (SHAP analysis), critical and positively correlated features can be extracted or selected to accelerate the design and synthesis of new materials. ML-assisted analysis using literature datasets was employed to reveal the multiple roles of various physicochemical features on enhancing the SC properties of ONPC materials. Deeper insights into the structure-activity relationship of ONPC materials were obtained by assessing the impacts of different features on each other and on the output variable. A PCC matrix was utilized to provide an overview of the correlation analysis among variables in the dataset. Some physicochemical characteristics of ONPC materials exhibited weak correlations with SC (r < 0.3) in the PCC matrix [Figure 1A], suggesting that the relationship between these specific characteristics and capacitive performance cannot be adequately explained by a linear function. This also indicates that there exists an interdependence among different physicochemical attributes for enhancing SC. A subset of 12 features (excluding strongly correlated variables such as Smic, Vmic, and Vt from the original 15 feature values) showing high correlation with pore structure features and capacitance were selected for modeling. The optimized ML model highlighted in Supplementary Figure 3 shows the RF model prediction outcomes (R2 = 0.86, RMSE = 17.11, and MAE = 12.78). Additionally, RF has considered the impact of all features comprehensively, as illustrated by the SHAP waterfall diagram of the model. The importance of 12 features was ranked through SHAP analysis [Figure 1B], highlighting the marginal contribution of each feature to the output using SHAP values. Figure 1B revealed that these parameters, such as SSA, N-Q%, N%, C=O%,
Figure 1. ML-assisted Analytics. (A) Heatmap of between features and SC (The colors of squares in the heat map represent the correlation's strength and direction); (B) SHAP waterfall diagram of feature importance; (C) Marginal contribution of twelve input features to the output variables based on a RF model. ML: Machine learning; SC: Specific capacitance; SHAP: SHapley Additive explanation; RF: Random forest.
Through ML-assisted interpretability analysis to optimize key features and rational design aided by in-situ catalytic pyrolysis, we successfully synthesized an ONPC material engineered for the efficient N and O doping sites, high SSA and utilization of graphene-like folding and amorphous hybrid. The process of synthesizing ONPC materials was depicted in Figure 2A. Essentially, ONPC materials were prepared using pre-treated lignite as a carbon precursor and oxygen source, g-C3N4 as a template, carbon precursors, and nitrogen source. This synthesis involves a straightforward ball-milling process and high-temperature pyrolysis, with the addition of K2CO3. The addition of K2CO3 serves a dual purpose: facilitating the
Figure 2. (A) Schematic illustration of synergistic fabricated ONPC materials; (B-D) SEM images of ONPC-700, ONPC-800, and ONPC-900, respectively. TEM images of ONPC-900; (D) low resolution; (E-G) high resolution, and (H) element mapping of C, N, and O. ONPC: O, N co-doped hierarchical porous carbon; SEM: Scanning electron microscope; TEM: Transmission electron microscope.
Subsequent analysis of nitrogen adsorption-desorption curves provided insight into the microscopic pore structure of the ONPC materials. It is important to highlight that the deliberately designed K2CO3 played a significant role in regulating the pore structure and balancing the amorphous and graphitized hybrid structure of the PCMs, thereby enhancing its energy storage capabilities. The isothermal adsorption/desorption curves of ONPC materials demonstrated a combination of type I and IV characteristics in Figure 3A, indicating the existence of micro and mesoporous. The PSD from 0.4 nm to 4 nm [Figure 3B] further confirmed the hierarchical pore structure of the ONPC materials, with micropores being predominant and small mesopores coexisting. A comparative analysis of micropore surface area, mesopore surface area, and micropore volume ratio (Vmic/Vt) was presented in Figure 3C. The comparison of
Figure 3. Pore structural characteristics of ONPC materials. (A) Nitrogen adsorption-desorption curves; (B) Pore-size distribution; (C) Specific surface area of mesopore and micropore and micropore volume ratio (Vmic/Vt). ONPC: O, N co-doped hierarchical porous carbon.
The carbonization transition of g-C3N4 by in-situ thermal exfoliation coincided with the emergence of
Figure 4. Composition and surface analysis of ONPC materials. (A) FTIR spectra; (B) Raman spectra; (C) Full XPS survey; High-resolution XPS spectra of (D) C 1s, (E) N 1s, and (F) O1s; (G) Relative content of C, N, and O; (H) Relative content of O species; (I) Relative content of N species. ONPC: O, N co-doped hierarchical porous carbon; FTIR: Fourier transforms infrared; XPS: X-ray photoelectron spectroscopy.
Additionally, the high-slope curve in the low-angle area of purified ONPC materials in Supplementary Figure 8B suggested that there are numerous micropores, consistent with the results of Figure 3B. Raman spectroscopy revealed two prominent peaks around 1,340 cm-1 and 1,593 cm-1 [Figure 4B], respectively, corresponding to the D band and the G band, respectively[39]. The D-band and G-band intensity ratio (ID/IG) of ONPC-900, standing at 1.18, surpassed those of OPC-900 (1.02) and NPC-900 (1.13), indicating that more defects and pores formation are caused by N and O co-doping into the ONPC-900 framework. The
Driven by the hierarchical porous structures with a high SSA, significant pore volume, and abundant active species such as N-5 and -COOH, along with a sub-nanopore dominated, interconnected graphene-like fold layered on pre-treated lignite derived carbon backbones, ONPC-900 exhibited superior electrochemical performance for supercapacitor. CV plots of obtained ONPC materials exhibited distorted
Figure 5. Electrochemical properties of ONPC materials in a three-electrode configuration. (A) CV plots of ONPC-900 measured at various scans (5~500 mV s-1); (B) GCD curves of ONPC-900 measured at various current densities; (C) GCD profiles of ONPC materials; (D) CV curves of ONPC materials; (E) SC comparison of ONPC materials measured at different current densities; (F) Nyquist plots of ONPC materials, inside is the zoomed high-frequency region of ONPC materials; (G) Comparison of the SC with these reported coal-derived carbon materials or ONPC materials[41,44-61]; (H) Radar chart for comparison of seventeen structural and test features and SC (F g-1) of all ONPC materials based on experiment in this work. In particular, the high grades in Dave (average pore diameter) mean low values. ONPC: O, N co-doped hierarchical porous carbon; CV: Cyclic voltammetry; GCD: Galvanostatic charge-discharge.
Based on the analysis of physicochemical features and electrochemical dynamics of the three-electrode system, it appears that active species of N, O co-doped have significant effects on the increase of total SC. Therefore, the theoretical analysis of independent adsorption species was carried out. Given the notable obvious differences in electrolyte affinity and the difficulty of K+ adsorption, all N-containing or
Figure 6. K+ adsorption on the surface of undoped, O-species and N-species doped carbon. (A) Adsorption of K+ on an ideal carbon surface (Precursor); (B-D) K+ adsorption on carbon surfaces doped with different O-doping functional groups; (E-G) Adsorption model of K+ on doped surfaces with different N functional groups.
Inspired by SHAP analysis of positive feature variables (SSA, -COOH, etc.) and structure adsorption sites (N or O doping species), and successful synthesis of ONPC electrode materials, a theoretical adsorption model was established subsequently to evaluate the synergistic effect of multiple physicochemical factors. To simulate a real O, N doped porous carbon surface, the ONPC-900 carbon surface with excellent performance was chosen for K+ adsorption based on XPS and pore-size distribution results (mainly distributed in 0.5~0.6 nm, corresponding to the analysis results in Figure 3B, as illustrated in Figure 7. Compared to an ideal carbon model (Figure 7A, Ea = -136.55 kJ mol-1), the adsorption energy of an undoped defect model on the carbon surface (Figure 7B-C, Ea = -401.76 kJ mol-1, Ea = -378.39 kJ mol-1) and
Figure 7. Models of ONPC-900 surface by DFT calculation. The adsorption models for K+ are (A) on an ideal carbon surface; (B-C) on an undoped defect carbon surface; (D-I) on various O, N co-doped defect carbon surfaces, respectively. ONPC: O, N co-doped hierarchical porous carbon; DFT: Density functional theory.
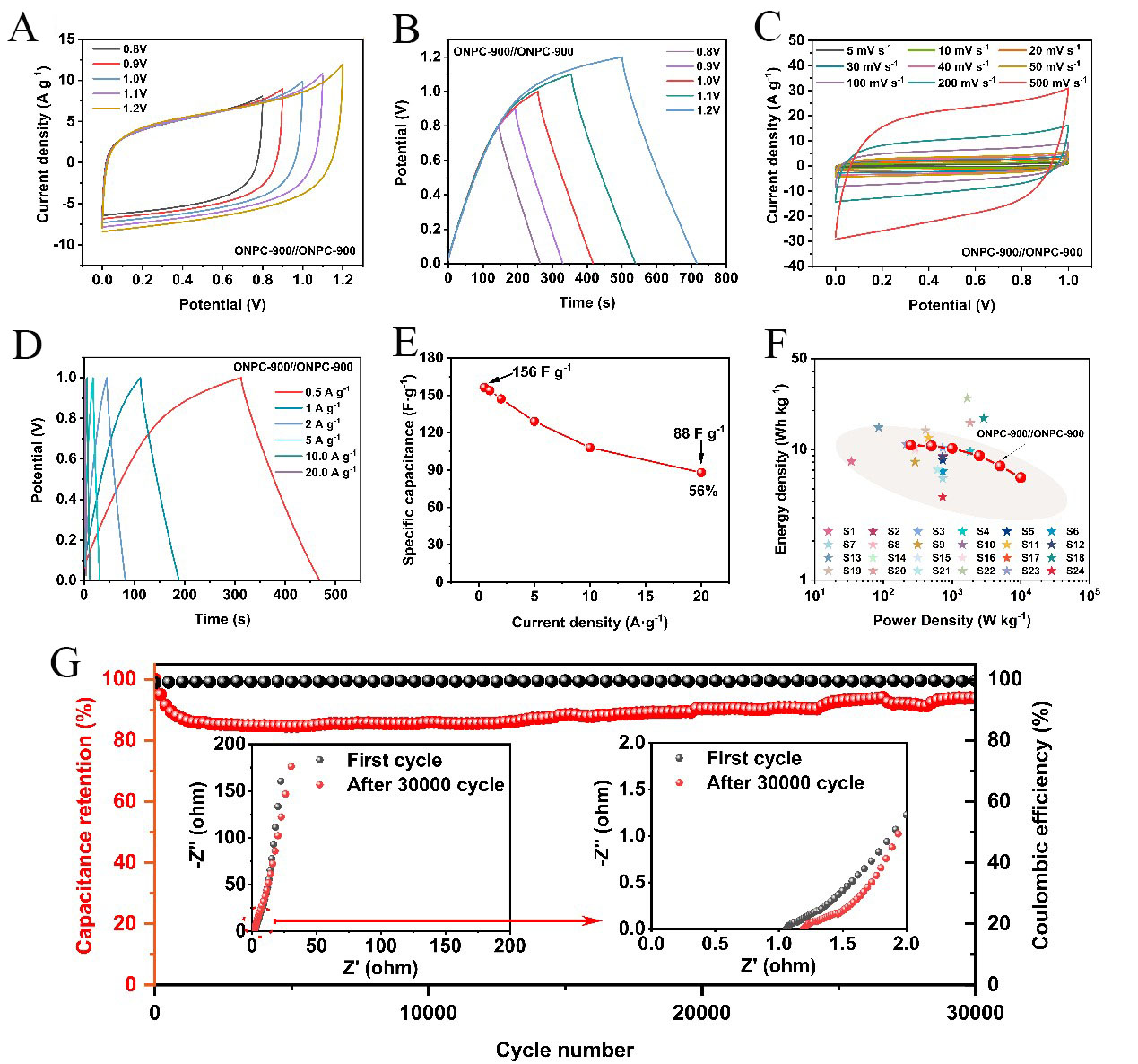
Considering the physicochemical properties of ONPC-900 and its excellent electrochemical performance, its practical application in electrode material of supercapacitors was also examined. To explore its application potential at a device level, 6 M KOH as an electrolyte was used to assemble a symmetric supercapacitor (ONPC-900//ONPC-900). Firstly, the CV testing [Figure 8A] of the ONPC-900//ONPC-900 symmetric supercapacitor was recorded at 100 mV s-1 and a maximum voltage range varying from 0.8 V to 1.2 V. The CV curves appeared as quasi-rectangular in the voltage window from 0 to 1.0 V, indicating electrochemical stability. Deviations from this shape beyond 1.0 V suggest interactions between the ionic species of the electrolyte and surface functionalities (carbonyl, carboxyl, phenol/benzene hydroxyl, etc.) on the carbon electrode. This working voltage range was further confirmed by GCD studies, where the
Figure 8. Electrochemical performances of the assembled symmetric supercapacitor by ONPC-900//ONPC-900 in a two-electrode system. (A) CV curves and (B) GCD curves at a scan rate of 100 mV s-1 with various current potential windows, respectively; (C) CV curves were measured at various scan rates (5-500 mV s-1); (D) GCD profiles were measured at different current densities (0.5-20
CONCLUSIONS
In summary, SHAP analysis using an emerging ML strategy revealed that the key physicochemical features of SSA and N-5, -COOH functional groups played positive effects in enhancing the capacitance performance in the complex structure-activity relationship. Through ML-assisted interpretability analysis to optimize key features and rational design, the controllable synthesis of ONPC materials was empirically validated via K2CO3-assisted pyrolysis, emphasizing the crucial contributions of in-situ exfoliation and etching pore generation. Underpinned by this synergistic design, the resulting ONPC-900 with the synergistic dominant structure retained the amorphous and the graphitized fold features. The hierarchical porous structure of ONPC-900 enriched with abundant sub-nanopores and N-5, -COOH active species, along with high O, N co-doping and graphitization. The analysis of physicochemical features and electrochemical characterization results revealed that the strategic co-doping of N and O within the inner pore defect and high SSA carefully customized the electronic structure of ONPC-900, which promoted ion adsorption and charge transfer and thus promoted the capacitive performance. DFT calculation further confirmed that the synergy of specific N-5, -COOH active sites and internal pore defects functionalized PCMs. Grounded in the adsorption model with synergistic dual-site internal defects, ONPC-900 demonstrated excellent capacitance storage performance. Specifically, the ONPC-900 exhibited a notable SC of 440 F g-1 at 0.5 A g-1 in a three-electrode system, and symmetric supercapacitors assembled by ONPC-900 demonstrated an exceptional long-term cycle stability of 30,000 cycles and a high-capacity retention rate of 93.8%. Additionally, the material showcased a substantial power density of 250 W kg-1 and a maximum energy density of 10.8 Wh kg-1. This work not only underscores the optimal design and controlled synthesis of PCMs with the synergistic structure of double-site and porous defect via regulatory structural engineering, but also improves the capacitive properties of porous carbon. Importantly, an emerging ML and DFT-assisted mechanism interpretation and verification strategy offers new avenues for the integrated structure-activity design of high-performance electrode materials.
DECLARATIONS
Authors’ contributions
Conceived the idea and designed the project: Liu, H.; Wang, Y.
Performed data analysis and interpretation: Liu, H.; Cui, Z.; Bai, Q.
Supervised the project: Liu, H.; Cui Z.; Qiao, Z.; Zhang, Y.
Drafted the manuscript: Liu, H.
Revised and finalized the manuscript: Liu, H.; Cui, Z.; Qiao, Z.; Zhang, Y.;, Bai, Q.; Wang, Y.
All authors read and approved the final manuscript.
Availability of data and materials
The data supporting this article have been included as part of the Supplementary Materials.
Financial support and sponsorship
The work was supported by the National Natural Science Foundation of China (No. 52371231), the Key R&D Program of Shanxi Province (No.202302040201008), and the Central Government Guides Local Science and Technology Development Special Fund Projects (No. YDZJSX2022B003).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Xu, M.; Zhu, X.; Lai, Y.; et al. Production of hierarchical porous biocarbon based on deep eutectic solvent fractionated lignin nanoparticles for high-performance supercapacitor. Appl. Energy. 2024, 353, 122095.
2. Yang, J.; Su, F.; Liu, T.; Zheng, X. Heteroatoms co-doped multi-level porous carbon as electrode material for supercapacitors with ultra-long cycle life and high energy density. Diamond. Relat. Mater. 2024, 141, 110693.
3. Sriram, G.; Hegde, G.; Dhanabalan, K.; et al. Recent trends in hierarchical electrode materials in supercapacitor: synthesis, electrochemical measurements, performance and their charge-storage mechanism. J. Energy. Storage. 2024, 94, 112454.
4. Sharma K, Arora A, Tripathi S. Review of supercapacitors: materials and devices. J. Energy. Storage. 2019, 21, 801-25.
5. Mansuer, M.; Miao, L.; Qin, Y.; et al. Trapping precursor-level functionalities in hierarchically porous carbons prepared by a pre-stabilization route for superior supercapacitors. Chinese. Chem. Lett. 2023, 34, 107304.
6. Guo, Z.; Han, X.; Zhang, C.; et al. Activation of biomass-derived porous carbon for supercapacitors: a review. Chinese. Chem. Lett. 2024, 35, 109007.
7. Jiang, G.; Senthil, R. A.; Sun, Y.; Kumar, T. R.; Pan, J. Recent progress on porous carbon and its derivatives from plants as advanced electrode materials for supercapacitors. J. Power. Sources. 2022, 520, 230886.
8. Da, S. L. M.; Cesar, R.; Moreira, C. M.; et al. Reviewing the fundamentals of supercapacitors and the difficulties involving the analysis of the electrochemical findings obtained for porous electrode materials. Energy. Storage. Mater. 2020, 27, 555-90.
9. Yang, N.; Ji, L.; Fu, H.; et al. Hierarchical porous carbon derived from coal-based carbon foam for high-performance supercapacitors. Chinese. Chem. Lett. 2022, 33, 3961-7.
10. Dong, K.; Sun, Z.; Jing, G.; et al. Nanoarchitectonics of self-supporting porous carbon electrode with heteroatoms co-doped: for high-performance supercapacitors. J. Energy. Storage. 2024, 85, 111048.
11. Li, H.; Li, Y.; Li, Y.; et al. Facile synthesis of heteroatom-doped hierarchical porous carbon with small mesopores for high-performance supercapacitors. J. Energy. Storage. 2024, 77, 110000.
12. Yuan, C.; Xu, H.; A. El-khodary S, et al. Recent advances and challenges in biomass-derived carbon materials for supercapacitors: a review. Fuel 2024, 362, 130795.
13. Zhang, R.; Liu, H.; Cui, Z.; Zhang, Y.; Wang, Y. Oxygen-rich microporous carbon derived from humic acid extracted from lignite for high-performance supercapacitors. Fuel 2024, 364, 131062.
14. Liu, H.; Wang, Y.; Lv, L.; Liu, X.; Wang, Z.; Liu, J. Oxygen-enriched hierarchical porous carbons derived from lignite for high-performance supercapacitors. Energy 2023, 269, 126707.
15. Peng, Y.; Chen, Z.; Zhang, R.; et al. Oxygen-containing functional groups regulating the carbon/electrolyte interfacial properties toward enhanced K+ storage. Nanomicro. Lett. 2021, 13, 192.
16. Chen, G.; Liu, Z.; Yang, G.; et al. Synthesis of chain-like nitrogen-doped carbon for high-performance supercapacitors. Colloids. Surf. A:. Physicochem. Eng. Asp. 2024, 687, 133498.
17. Hajibaba, S.; Gholipour, S.; Pourjafarabadi, M.; et al. Electrochemical sulfur-doping as an efficient method for capacitance enhancement in carbon-based supercapacitors. J. Energy. Storage. 2024, 79, 110044.
18. Suman, S.; Ficek, M.; Sankaran, K. J.; et al. Nitrogen-incorporated boron-doped diamond films for enhanced electrochemical supercapacitor performance. Energy 2024, 294, 130914.
19. Kim, D.; Jin, X.; Cho, Y.; et al. Facile preparation of N-doped porous carbon nanosheets derived from potassium citrate/melamine for high-performance supercapacitors. J. Electroanal. Chem. 2021, 892, 115302.
20. Liu, A.; Yan, L.; Zhang, Y.; et al. Nitrogen-doped coal-based microporous carbon material co-activated by HCOOK and urea for high performance supercapacitors. Surf. Interfaces. 2024, 44, 103754.
21. Farma, R.; Apriyani, I.; Awitdrus; et al. Enhanced electrochemical performance of oxygen, nitrogen, and sulfur trial-doped Nypa fruticans-based carbon nanofiber for high performance supercapacitors. J. Energy. Storage. 2023, 67, 107611.
22. Zhang, G.; Zhang, Y.; Wang, J.; et al. Nitrogen-functionalization of carbon materials for supercapacitor: Combining with nanostructure directly is superior to doping amorphous element. J. Colloid. Interface. Sci. 2024, 660, 478-89.
23. Hou, L.; Yang, W.; Li, Y.; et al. Dual-template endowing N, O co-doped hierarchically porous carbon from potassium citrate with high capacitance and rate capability for supercapacitors. Chem. Eng. J. 2021, 417, 129289.
24. Cai, L.; Zhang, Y.; Ma, R.; et al. Nitrogen-doped hierarchical porous carbon derived from coal for high-performance supercapacitor. Molecules 2023, 28, 3660.
25. Zahra, T.; Gassoumi, A.; Gouadria, S.; et al. Facile fabrication of BiFeO3/g-C3N4 nanohybrid as efficient electrode materials for supercapacitor application. Diamond. Relate. Mater. 2024, 144, 110927.
26. Yang, K.; Fan, Q.; Song, C.; et al. Enhanced functional properties of porous carbon materials as high-performance electrode materials for supercapacitors. Green. Energy. Resour. 2023, 1, 100030.
27. Kolavada, H.; Gajjar, P.; Gupta, S. K. Unraveling quantum capacitance in supercapacitors: energy storage applications. J. Energy. Storage. 2024, 81, 110354.
28. Shah, S. S.; Aziz, M. A.; Ali, M.; Hakeem, A. S.; Yamani, Z. H. Advanced high-energy all-solid-state hybrid supercapacitor with nickel-cobalt-layered double hydroxide nanoflowers supported on jute stick-derived activated carbon nanosheets. Small 2024, 20, e2306665.
29. Reddy, B.; Narayana, P.; Maurya, A.; et al. Modeling capacitance of carbon-based supercapacitors by artificial neural networks. J. Energy. Storage. 2023, 72, 108537.
30. Liu, P.; Wen, Y.; Huang, L.; et al. An emerging machine learning strategy for the assisted‐design of high-performance supercapacitor materials by mining the relationship between capacitance and structural features of porous carbon. J. Electroanal. Chem. 2021, 899, 115684.
31. Rahimi, M.; Abbaspour-fard, M. H.; Rohani, A. Synergetic effect of N/O functional groups and microstructures of activated carbon on supercapacitor performance by machine learning. J. Power. Sources. 2022, 521, 230968.
32. Saad, A. G.; Emad-eldeen, A.; Tawfik, W. Z.; El-deen, A. G. Data-driven machine learning approach for predicting the capacitance of graphene-based supercapacitor electrodes. J. Energy. Storage. 2022, 55, 105411.
33. Krishnan, A.; Yoosuf, M.; Archana, K.; A. s. A, Viswam A. Metal derivative (MD)/g-C3N4 association in hydrogen production: a study on the fascinating chemistry behind, current trend and future direction. J. Energy. Chem. 2023, 80, 562-83.
34. Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. Condens. Matter. 1996, 54, 11169-86.
35. Jha, S.; Yen, M.; Salinas, Y. S.; Palmer, E.; Villafuerte, J.; Liang, H. Machine learning-assisted materials development and device management in batteries and supercapacitors: performance comparison and challenges. J. Mater. Chem. A. 2023, 11, 3904-36.
36. Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: a review. Mater. Des. 2023, 230, 111952.
37. Thi, Q. H.; Man, P.; Huang, L.; Chen, X.; Zhao, J.; Ly, T. H. Superhydrophilic 2D carbon nitrides prepared by direct chemical vapor deposition. Small. Sci. 2023, 3, 2200099.
38. Yang, H.; Lin, H.; Yang, C.; et al. Structural regulation of carbon materials through hydrothermal mixing of biomass and its application in supercapacitors. J. Energy. Storage. 2024, 83, 110688.
39. Yang, B.; Zhang, D.; Li, Y.; et al. Locally graphitized biomass-derived porous carbon nanosheets with encapsulated Fe3O4 nanoparticles for supercapacitor applications. Chem. Eng. J. 2024, 479, 147662.
40. Ran, F.; Yang, X.; Xu, X.; Li, S.; Liu, Y.; Shao, L. Green activation of sustainable resources to synthesize nitrogen-doped oxygen-riched porous carbon nanosheets towards high-performance supercapacitor. Chem. Eng. J. 2021, 412, 128673.
41. Liu, Z.; Qin, A.; Zhang, K.; Lian, P.; Yin, X.; Tan, H. Design and structure of nitrogen and oxygen co-doped carbon spheres with wrinkled nanocages as active material for supercapacitor application. Nano. Energy. 2021, 90, 106540.
42. Raha, H.; Pradhan, D.; Guha, P. K. Ultrahigh coulombic efficiency in alkali metal incorporated biomass derived carbon electrode. J. Electroanal. Chem. 2023, 931, 117193.
43. Song, Z.; Li, L.; Zhu, D.; et al. Synergistic design of a N, O co-doped honeycomb carbon electrode and an ionogel electrolyte enabling all-solid-state supercapacitors with an ultrahigh energy density. J. Mater. Chem. A. 2019, 7, 816-26.
44. Le, F.; Ren, P.; Jia, W.; Wang, T.; Tao, Y.; Wu, D. High-yield preparation of coal tar pitch based porous carbon via low melting point fire retardant carbonation strategy for supercapacitor. Chem. Eng. J. 2023, 470, 144131.
45. Yang, Y.; Zuo, P.; Qu, S. Adjusting hydrophily and aromaticity strategy for pitch-based hierarchical porous carbon and its application in flexible supercapacitor. Fuel 2022, 311, 122514.
46. Zhang, H.; Sun, X.; Zheng, Y.; Zhou, J. Scalable synthesis of N, O co-doped hierarchical porous carbon for high energy density supercapacitors. J. Colloid. Interface. Sci. 2024, 658, 1025-34.
47. Li, W.; Li, C.; Xu, Y.; et al. Heteroatom-doped and graphitization-enhanced lignin-derived hierarchically porous carbon via facile assembly of lignin-Fe coordination for high-voltage symmetric supercapacitors. J. Colloid. Interface. Sci. 2024, 659, 374-84.
48. Zhang, Y.; Zheng, H.; Wang, Q.; et al. 3-Dimensional porous carbon derived from waste aucklandia lappa straw for high-performance liquid and all-solid-state supercapacitors. J. Electroanal. Chem. 2024, 953, 117992.
49. Zheng, L.; Dai, X.; Ouyang, Y.; Chen, Y.; Wang, X. nHighly N/O co-doped carbon nanospheres for symmetric supercapacitors application with high specific energy. J. Energy. Storage. 2021, 33, 102152.
50. Ma, T.; Xu, S.; Zhu, M. Porous carbon from verbena straw with self-doped O/N and its high-performance aqueous and flexible all-solid-state supercapacitors. J. Power. Sources. 2024, 597, 234147.
51. Shaku, B.; Mofokeng, T.; Coville, N.; Ozoemena, K.; Maubane-nkadimeng, M. Biomass valorisation of marula nutshell waste into nitrogen-doped activated carbon for use in high performance supercapacitors. Electrochim. Acta. 2023, 442, 141828.
52. Feng, L.; Wang, M.; Chang, Y.; et al. Polymerization-Pyrolysis-Derived Hierarchical Nitrogen-Doped Porous Carbon for Energetic Capacitive Energy Storage. ACS. Appl. Energy. Mater. 2023, 6, 7147-55.
53. Wang, T.; Guo, J.; Guo, Y.; Feng, J.; Wu, D. Nitrogen-Doped Carbon Derived from Deep Eutectic Solvent as a High-Performance Supercapacitor. ACS. Appl. Energy. Mater. 2021, 4, 2190-200.
54. Dong, D.; Zhang, Y.; Xiao, Y.; Wang, T.; Wang, J.; Gao, W. Oxygen-enriched coal-based porous carbon under plasma-assisted MgCO3 activation as supercapacitor electrodes. Fuel 2022, 309, 122168.
55. Zhang, Z.; Li, Y.; Yang, X.; et al. In-situ confined construction of N-doped compact bamboo charcoal composites for supercapacitors. J. Energy. Storage. 2023, 62, 106954.
56. Park, S.; Seo, B.; Shin, D.; Kim, K.; Choi, W. Sodium-chloride-assisted synthesis of nitrogen-doped porous carbon shells via one-step combustion waves for supercapacitor electrodes. Chem. Eng. J. 2022, 433, 134486.
57. Li, G.; Chen, S.; Wang, Y.; Wang, G.; Wu, Y.; Xu, Y. N, S co-doped porous graphene-like carbon synthesized by a facile coal tar pitch-blowing strategy for high-performance supercapacitors. Chem. Phys. Lett. 2023, 827, 140712.
58. Yang, X.; Sun, G.; Wang, F.; et al. Rational design of dense microporous carbon derived from coal tar pitch towards high mass loading supercapacitors. J. Colloid. Interface. Sci. 2023, 646, 228-37.
59. Dong, D.; Zhang, Y.; Xiao, Y.; et al. High performance aqueous supercapacitor based on nitrogen-doped coal-based activated carbon electrode materials. J. Colloid. Interface. Sci. 2020, 580, 77-87.
60. Dong, D.; Xiao, Y.; Xing, J. Facile wet mechanochemistry coupled K2FeO4 activation to prepare functional coal-derived hierarchical porous carbon for supercapacitors. J. Cleaner. Prod. 2023, 428, 139474.
61. Zhang, R.; Jing, X.; Chu, Y.; et al. Nitrogen/oxygen co-doped monolithic carbon electrodes derived from melamine foam for high-performance supercapacitors. J. Mater. Chem. A. 2018, 6, 17730-9.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.



























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].