Artificial intelligence in laparoscopic cholecystectomy: does computer vision outperform human vision?
Abstract
Background: The occurrence of biliary duct injury (BDI) after laparoscopic cholecystectomy (LC) remains 0.2-1.5%, which is largely caused by anatomic misidentifications. To solve this problem, we developed an artificial intelligence model, SurgSmart, and preliminarily verified its potential surgical guidance ability by comparing its performance with surgeons.
Methods: We prospectively collected 60 LC videos from November 2019 to August 2020 and enrolled 41 videos into the model establishment. Four important anatomic regions, namely cystic duct, cystic artery, common bile duct, and cystic plate, were annotated, and YOLOv3 (You Look Only Once), an object detection algorithm, was applied to develop the model SurgSmart. To further evaluate its performance, comparisons were made among SurgSmart, trainees, and seniors (surgical experience in LC > 100).
Results: In total, 101,863 frames were extracted from videos, and 5533 video frames were selected, annotated, and used in model training. The mean average precision (mAP) of SurgSmart was 0.710. Comparative results show SurgSmart had significantly higher intersection-over-union (IoU) and accuracy (IoU ≥ 0.5) in anatomy detection than those of seniors (n = 36) and trainees (n = 32) despite the existence of severe inflammation. Additionally, SurgSmart tended to correctly identify anatomic regions in earlier surgical phases than most of the seniors and trainees (P < 0.001).
Conclusions: SurgSmart is not only capable of accurately detecting and positioning anatomic regions in LC but also has better performance than that of the trainees and seniors in terms of individual still images and the whole set.
Keywords
INTRODUCTION
Laparoscopic cholecystectomy (LC), one of the most frequent minimally invasive surgeries performed worldwide, has become a gold standard for patients with symptomatic gallstones[1]. Although minimally invasive techniques have been developing for decades, biliary duct injury (BDI), which leads to re-interventions, increased mortality, and worsened quality of life[2-4], remains one of the most severe complications for LC. To mitigate BDI, several safe intraoperative approaches, such as critical view of safety (CVS), intraoperative biliary imaging, and subtotal cholecystectomy, have been recommended in various circumstances during LC[5,6]. Despite these preventive approaches, the overall incidence of BDI is still unchanged for many years and ranges from 0.2% to 1.5% globally[7,8]. Such phenomenon is mainly due to the widely varying experience and anatomic localization ability of different surgeons. In an international survey involving over 600 surgeons, 72.3% of the surgeons had experienced BDI or near-misses, nearly half of whom acknowledged that the misrecognition of anatomic landmarks was the leading cause of BDI, especially between cystic duct and common bile duct[9]. Hence, appropriately identifying pivotal structures including cystic duct, cystic artery, and common bile duct through anatomic guidance in real time is urgently needed.
Meanwhile, computer vision, a subfield of artificial intelligence (AI), has witnessed a dramatic resurgence in the past few years due to increases in computational power and the availability of big datasets[10]. These technologies focus on image and video analysis, dealing with tasks such as object classification, detection, and segmentation[11]. Meanwhile, medical images have greatly benefited from recent advances in computer vision. Many studies have indicated the promising results of computer vision in complex diagnostics such as dermatology[12], radiology[13], pathology[14], and so on. These models were then mostly proved to be non-inferior or even superior to clinicians[15,16]. Simultaneously, analysis of surgical videos, which contain a large amount of visible intraoperative information[17], is another pivotal application scenario for computer vision. With the significant improvement in the efficiency and accuracy of the neural network[18], more than 30 studies have demonstrated that computer vision could process a massive number of intraoperative videos to gain valuable information[19]. For LC, computer vision could automatically identify the critical anatomic regions mentioned above[20-22].
Therefore, in this study, we developed the computer vision model (SurgSmart) for LC and preliminarily verified its potential surgical guidance ability by comparing its performance with trainees and seniors.
METHODS
LC videos screening and the construction of anatomic dataset
From November 2019 to August 2020, we prospectively collected 60 LC videos in West China Hospital, Sichuan University, China. This study was approved by the Ethics Committee Board of our hospital. After excluding videos that were incomplete (n = 1), pixels or format incompatible (n = 17), or gallbladder gangrene (n = 1), 41 LC videos were included in the model development. Assigned by admission number and date of surgery, video frames were then extracted from videos included (one frame per second). Among these, ineligible frames (out-of-site, blurred, or duplicated) were excluded manually [Figure 1]. The remaining frames were then distributed and annotated by nine well-trained surgeons with over 100 LC video reviewing experiences[23-25]. To ensure the accuracy and reliability of the annotation, the supervisor reviewed the annotators’ work and made corrections if there was any mistake. Four important anatomic regions, namely cystic duct, cystic artery, common bile duct, and cystic plate, were annotated by bounding boxes [Supplementary Material 1]. All the work was conducted through Anaconda (Anaconda 2019, Inc, Austin, TX) in Python 3.6.5. NVIDIA (NVIDIA, Santa Clara, CA).
Algorithm development of SurgSmart
YOLOv3, an algorithm for object detection, was applied to develop SurgSmart. It consists of a residual network and a feature pyramid network. The residual network plays the role of improving processing efficiency, and the function of the feature pyramid network is feature extraction and multi-scale and multi-target detection [Supplementary Material 2][26]. Boosting scene understanding and multiple object detection ability of the model, the COCO dataset (a dataset provided by Microsoft used for image recognition) was used for pre-training and transfer learning[27]. Meanwhile, multi-scale inputs for data augmentation were used to improve the performance of our model. Tesla V100 Graphic Processing Unit (GPU) was used for training, validation, and testing. Additionally, 80%, 10%, and 10% of the annotated frames from different videos were assigned into training, validation, and testing datasets, respectively [Figure 1]. The training and validation datasets came from the West China Hospital, and the test dataset came from its healthcare alliance.
SurgSmart model evaluation
Defined as the area of the intersection divided by the area of union [Supplementary Material 3a], intersection over union (IoU) was applied to determine the results of anatomic identification. The performance of SurgSmart was evaluated by precision {Precision = [(True Positive/(True Positive + False Positive)]} recall {Recall = [(True Positive/(True Positive + False Negative)]}, and average precision. Average precision (AP) is defined as the area under the precision-recall curve [Supplementary Material 3b]. Additionally, the mean average precision (mAP), which is the arithmetic mean of AP, was applied to evaluate the overall performance of SurgSmart.
Comparison between SurgSmart and trainees/seniors
To further evaluate the model’s performance, we compared the anatomic recognitional ability of SurgSmart, trainees, and seniors. Trainees were defined as interns who had watched < 100 LC videos previously but without clinical experience, whereas seniors were defined as surgical fellows and attendings with > 100 LC operation experiences[23,24]. The evaluation included marking three anatomic regions (cystic duct, cystic artery, and common bile duct) from 28 intraoperative still images from two LC videos, with corresponding time during video clips marked. Among them, 15 video pictures came from a video with low disease severity (Parkland Grade ≤ 2), and the others came from another video with a high inflammatory level (Parkland Grade ≥ 3).[28] Anatomic identification results were evenly evaluated by dividing all the images into 7 × 5 equal parts and measured by IoU [Figure 2], which indicates correct identification if no less than 0.5. Apart from comparing the correct identification rate on the still image level, we also evaluated the identification rate of three groups through the chronological level, which means identifying correctly one image in its whole set could be recognized as correctness. Furthermore, the time point (marked on each picture using the above method) of the earliest correct identification of each anatomic region was recorded and compared among the three groups.
Figure 2. An example of judging detection IoU of human surgeon. The standard answer for common bile duct is “d4:e5” (shown in the blue rectangle with shadow). If the surgeon chose an answer(s), which could not form a rectangle, such as choosing e2, e3, f3, d4, and f4 (shown in yellow tick) simultaneously, it was converted into the horizontal rectangle to include the answer(s) below. Therefore, the IoU would be {[Intersection (shown in green shadow)/Union = 1/9 ≈ 0.111]} in this circumstance.
Statistical analysis
We analyzed the comparison results through SPSS Statistics version 23.0 (IBM Corp., Armonk, NY). Cronbach’s alpha and Kaiser-Meyer-Olkin and Bartlett’s test of the results were used respectively to evaluate the reliability and construct validity of the test[29,30]. Numeric data, IoU, were represented as 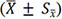 and compared between SurgSmart and trainees/seniors through one-sample t-tests, and they were compared between trainees and seniors through independent samples t-test. For ranked data, we used Wilcoxon signed-rank test to compare detection time points and successful detection rate between SurgSmart and trainees/seniors and used the Mann-Whitney U test to compare them between trainees and seniors. The binominal test was used in comparisons of correct identification rates between SurgSmart and surgeons. Pa, Pb, and P’ stand for P-value between SurgSmart and trainees, SurgSmart and seniors, trainees and seniors, respectively. P < 0.05 (both sides) was considered as statistically significant, and 95% of confidential intervals were all two-sided.
and compared between SurgSmart and trainees/seniors through one-sample t-tests, and they were compared between trainees and seniors through independent samples t-test. For ranked data, we used Wilcoxon signed-rank test to compare detection time points and successful detection rate between SurgSmart and trainees/seniors and used the Mann-Whitney U test to compare them between trainees and seniors. The binominal test was used in comparisons of correct identification rates between SurgSmart and surgeons. Pa, Pb, and P’ stand for P-value between SurgSmart and trainees, SurgSmart and seniors, trainees and seniors, respectively. P < 0.05 (both sides) was considered as statistically significant, and 95% of confidential intervals were all two-sided.
RESULTS
We extracted 101,863 frames from 41 LC videos, and 5533 frames were manually selected and annotated according to the criteria. There were 4427 (80%), 553 (10%), and 553 (10%) images randomly assigned to training, validation, and test datasets, respectively. The demographic characteristics of 41 patients who have undergone LC are shown in Table 1, indicating most patients underwent elective surgery (37/41, 90.24%). Moreover, most patients were diagnosed with chronic calculous cholecystitis (n = 34, 82.93%) and only six (14.63%) patients with acute attacks.
The demographic character of the video cases enrolled in model establishment
| Factors | Statistical Results |
Operation Age (Years) 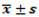 | 51.75 ± 11.09 |
| Gender, N (%) | |
| Male | 13 (31.71%) |
| Female | 28 (68.29%) |
| Ethnicity, N (%)# | |
| Han | 39 (95.12%) |
| Qiang | 1 (2.44%) |
| Miao | 1 (2.44%) |
| Surgery type, N (%) | |
| Elective | 37 (90.24%) |
| Urgent | 4 (9.76%) |
| Body Mass Index, kg/m2, N (%) | |
| ≤ 18.5 | 5 (12.20%) |
| 18.5-23.9 | 23 (56.10%) |
| 24.0-27.9 | 10 (24.39%) |
| ≥ 28.0 | 3 (7.31%) |
| History of Hypertension, N (%) | |
| Yes | 6 (14.63%) |
| No | 35 (85.37%) |
| History of Hepatic Disease, N (%)* | |
| Yes | 6 (14.63%) |
| No | 35 (85.37%) |
Hemoglobin (g/L), 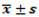 | 136.78 ± 14.45 |
| White Blood Cells, 109/L, M(Q25 -Q75) | 5.90 (4.50-7.75) |
| Alanine transaminase, IU/L, M(Q25 -Q75) | 23 (16-38) |
| Triglyceride, mmol/L, M(Q25 -Q75) | 1.30 (1.04-1.95) |
Cholesterol, mmol/L, 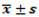 | 4.77 ± 0.89 |
| hs-CRP (mg/L), M(Q25 -Q75) | 1.43 (0.65-3.00) |
APTT (s), 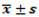 | 28.58 ± 1.88 |
| ASA Grading, N (%) | |
| 1 | 6 (14.63%) |
| 2 | 33 (80.49%) |
| 3 | 2 (4.88%) |
| Pathology Results, N (%) | |
| Chronic Calculous Cholecystitis | 34 (82.93%) |
| Acute Attack of Chronic Calculous Cholecystitis | 6 (14.63%) |
| Chronic cholecystitis with cholesterol polyps | 1 (2.44%) |
| Postoperative Hospitalization Days, M(Q25 -Q75) | 1 (1-3) |
For the performance of SurgSmart, the mAP of the four regions was 0.710. Specifically, the AP of the common bile duct, cystic duct, cystic artery, and the cystic plate was 0.855, 0.696, 0.504, and 0.787, respectively [Table 2].
Testing results of SurgSmart
| Metric | Mean Average Precision | Common bile duct | Cystic bed | Cystic duct | Cystic artery |
| Precision | 0.861 | 0.842 | 0.747 | 0.637 | |
| Recall | 0.905 | 0.848 | 0.784 | 0.632 | |
| Average Precision | 0.710 | 0.855 | 0.787 | 0.696 | 0.504 |
After excluding invalid responses due to the nullification, 68 surgeons were involved in the AI-surgeon comparison. Among them, 32 participants were classified as trainees, and 36 surgeons were classified as seniors. The Cronbach’s alpha is 0.817 and 0.844 before and after excluding Questions 4, 9, 10, 18, and 27, respectively [Supplementary Material 4], showing good internal consistency. The validity of the test indicates mediocre construct validity, Kaiser-Meyer-Olkin Test and Bartlett’s Test results are 0.620 and 779.540 (P < 0.001), respectively, before the exclusion and 0.658 and 602.200 (P < 0.001), respectively, after such exclusion [Supplementary Material 5].
For each still image, the mean IoU of SurgSmart, trainees, and seniors were 0.561, 0.299 ± 0.013, and 0.450 ± 0.013 in cases without severe inflammation (Pa, Pb, P’ < 0.001). The mean IoU of SurgSmart, trainees, and seniors were 0.646, 0.275 ± 0.019, and 0.407 ± 0.020, respectively, in cases with severe inflammation (Pa, Pb, P’ < 0.001). Over half of the images witnessed statistically significant disparity between pairs of SurgSmart, trainees, and seniors (P < 0.001) [Supplementary Material 6]. Furthermore, according to the IoU curves shown in Figure 3, despite the IoU of three groups fluctuating at different levels among different images, the shape of curves had some similarities, especially in the case with severe inflammation.
Figure 3. Comparison of overall detection results in frames of LC with and without severe inflammation: (A) frames from a low-severity case (Parkland Grade ≤ 2); and (B) frames from a high-severity case (Parkland Grade ≥ 3). CI, Coefficient Interval; IoU, Intersection over Union. The question numbers were arranged arbitrarily. Questions 4, 9, 10, 18, and 27 were excluded.
Then, we also compared correct anatomic identification rates among the three groups. As shown in Table 3, in the case without inflammation, the accuracy of each structure recognition for SurgSmart on still images ranged from 60% to 100%, whereas those for trainees and seniors were 25.52%-27.34% and 40.74%-59.03%, respectively (Pa, Pb, P’ ≤ 0.001). At chronological images level, the correct identification rates of each anatomic region were 88.89%-96.37% for seniors, and those of trainees were 56.25%-68.75% (Pa, Pb, P’ < 0.001). In cases with inflammation, the accuracy of anatomic regions for SurgSmart ranged from 66.67% to 100% on the still-image level, whereas those for trainees/seniors ranged from 8.33% to 75.93% (Pa, Pb < 0.001), the highest of which were those of common bile duct. On the chronological-images level, the correct identification rate of common bile duct for trainees and seniors was 68.75% and 94.44%, respectively (P’ = 0.006). All these results demonstrate that the accuracy of SurgSmart in anatomic regional identification was significantly higher than that of seniors and trainees (Pa, Pb < 0.001).
Comparison of successful detection rate of anatomic landmarks (threshold of IoU: 0.5)
| With severe inflammation | Category of successful detection Rate | Anatomic Parts | SurgSmart | Trianees | Pa | Seniors | Pb | P’ |
| No | Still images | Cystic duct | 4/4 (100.00%) | 35/128 (27.34%) | -- | 85/144 (59.03%) | -- | |
| Cystic artery | 5/6 (83.33%) | 49/192 (25.52%) | 88/216 (40.74%) | |||||
| Common bile duct | 3/5 (60.00%) | 52/160 (32.50%) | 96/180 (53.33%) | |||||
| Chronological | Cystic duct | -- | 18/32 (56.25%) | -- | 35/36 (96.37%) | -- | ||
| Cystic artery | -- | 22/32 (68.75%) | -- | 35/36 (96.37%) | -- | 0.002 | ||
| Common bile duct | -- | 21/32 (65.62%) | -- | 32/36 (88.89%) | -- | 0.002 | ||
| Yes | Still images | Cystic duct | 2/3 (66.67%) | 10/96 (10.42%) | 21/108 (19.44%) | 0.074 | ||
| Cystic artery | 2/2 (100.00%) | 8/64 (12.50%) | -- | 6/72 (8.33%) | -- | 0.427 | ||
| Common bile duct | 3/3 (100.00%) | 47/96 (48.96%) | -- | 82/108 (75.93%) | -- | |||
| Choronoglogical | Cystic duct | -- | 9/32 (28.12%) | -- | 21/36 (58.33%) | -- | 0.013 | |
| Cystic artery | -- | 8/32 (25.00%) | -- | 6/36 (16.67%) | -- | 0.4 | ||
| Common bile duct | 22/32 (68.75%) | 34/36 (94.44%) | 0.006 |
Additionally, comparison results of the earliest surgical phases of correct identification are depicted in Figure 4. Even though seniors tended to correctly identify the anatomic regions in an earlier phase than trainees (P’ < 0.01) except cystic artery in cases with severe inflammation, the performance of SurgSmart was superior to most of the surgeons regardless of experience (Pa, Pb < 0.01).
Figure 4. Comparison of detection time point of human surgeons and SurgSmart towards cystic duct and artery with and without severe inflammation: (A, B) for a low-severity case (Parkland Grade ≤ 2); and (C, D) for a high-severity case (Parkland Grade ≥ 3). *P < 0.05 between trainees/seniors and SurgSmart; **P < 0.01 between trainees/seniors and SurgSmart; ^P < 0.05 between trainees and seniors; ^^P < 0.01 between trainees and seniors. The red reference line stands for the detection time point of SurgSmart.
DISCUSSION
The ongoing development of computer vision technology, one of the areas of artificial intelligence, has made it possible to “recognize” images and videos with deep learning algorithms[18]. Significant advances in these fields have resulted in AI achieving human-level object detection and scene recognition[31,32]. As mentioned above, misrecognition of key anatomic regions such as the common bile duct is the main reason for BDI. Therefore, analyzing LC videos based on AI technologies may help us with clinical decision support and reduce iatrogenic injuries. However, AI-based surgical video analysis is still premature due to the complexity of the surgery and individual variance. In this study, we developed an AI-based anatomic recognition model, named SurgSmart, which could identify key anatomic regions of LC with a high degree of mAP (0.710). The anatomic recognitional performance comparison among SurgSmart, trainees, and seniors demonstrated that SurgSmart had a significantly higher IoU and correct anatomic identification rate than both trainees and seniors, regardless of disease severity. Moreover, SurgSmart also tended to correctly identify anatomic regions in earlier surgical phases during LC than most surgeons did.
Given that the misidentification of anatomic regions or landmarks during LC is the dominant factor contributing to BDI, correct recognition of these structures is fundamental for safe surgery[5]. Although computer vision, a subtype of AI technology, has shown quite promising results for various pattern and object recognition tasks in medicine[14,32], its application for real-time surgery is much more challenging since surgical videos have significantly more complexity in terms of background noise, anatomic regions, and various intraoperative information[33]. In early research, Sato et al.[34] explored the feasibility of ureter recognition of AI in 19 cases of laparoscopic hysterectomy. Although this study could not achieve satisfying results in detecting ureters using the Open-Source Computer Vision Library, the preliminary work still suggested the possibility of computer vision in assisting laparoscopic surgery. Later, Madad Zadeh et al.[35] used another deep learning method called Mask R-CNN to develop an algorithm named SurgAI. Its detection accuracy and segmentation accuracy of ureter were 97% and 84.5%, respectively, which further indicated the feasibility of AI-assisted anatomy recognition using the AI technique.
To mitigate the BDI rate of LC by AI technologies, several institutes have carried out relevant preliminary studies, some of which demonstrated promising results: Tokuyasu et al.[36] developed and validated an AI-assisted anatomy recognition system for LC. In this study, YOLOv3, an algorithm of objects detection, was applied to identify anatomic regions (such as cystic duct and common bile duct) and landmarks recognition [such as the lower edge of liver segment 4 (S4) and Rouviere’s sulcus]. Although the AP for each structure or landmark showed poor results, ranging from 0.074 to 0.320, the valid anatomic identification was confirmed on 22 of the 23 videos, demonstrating its great potential to mitigate BDI. In our study, four anatomic structures, directly or indirectly associated with CVS, were selected for model development. After amplifying the training datasets and optimizing the YOLOv3 model by sample equalization, the mAP of key anatomic regions was significantly increased to 0.710, even in cases with severe inflammation[28]. Meanwhile, other studies have explored anatomic evaluation approaches, such as safe/dangerous zone identification. For instance, Madani et al.[33] developed and validated the performance of an AI algorithm that could identify safe/dangerous zones based on the location within the hepatocystic triangle, with the potential for real-time guidance during LC. In addition, automatic evaluation of CVS, which is a gold standard of anatomic processing during LC, was also applied to reduce BDI[5]. Korndorffer et al.[21] developed an auto-evaluating model for CVS, which showed promising results, especially in cases with severe inflammation. Moreover, according to the latest research, Mascagni et al.[37] developed a deep learning model to automatically assess CVS during LC with a mean average precision of 71.9%, suggesting AI could be able to assess CVS criteria. Therefore, with the help of continuous dataset training and ongoing breakthroughs in computer algorithms, combined with multiple methods, such as anatomy detection/segmentation, CVS evaluation, safe-zones guidance, and more, AI systems will be applied for supporting the clinical decision and ensuring the safety during surgery.
To be noted, the significantly varied performance of AI recognition among different anatomic regions probably lies in their feature. To begin with, the size of the anatomic region has a significant influence on AI performance. In the studies conducted by Madad Zadeh et al.[35], the accuracy of large anatomic regions such as the uterus was 84.5% in contrast with those with small anatomy regions such as ovaries, only 29.6% in the SurgAI algorithm, indicating larger objects always have better recognition effects. Furthermore, a clear and regular boundary of anatomic regions will be conducive to deep learning. Structures such as the gallbladder and liver have significantly higher IoU when compared to dissected hepatocystic triangle and cystic plate[33,37]. Additionally, some anatomic regions with various intraoperative exposure conditions are difficult for AI recognition. In our study, the exposure state of “dynamic” structures such as cystic duct and cystic artery change constantly during the hepatocystic mobilization, while those of “non-dynamic” regions such as liver and common bile duct almost remain unchanged, leading to the variance of recognition performance in terms of AP. Thus, balanced training datasets and individualized algorithms for different anatomic regions might be essential for optimizing the overall capability of AI systems.
In 2017, Esteva et al.[12] suggested that deep learning achieved performance on par with all board-certificated dermatologists in terms of skin cancer diagnosis, indicating that AI can classify skin cancer comparable to experts. Later, several studies demonstrated that the AI systems performed at physician-like levels in lesions identification and classification, such as breast cancer detection in mammography[15], lung cancer diagnosis in CT[16], and others. Nevertheless, it is estimated that a 1 min high-quality surgical clip contains 25 times as many data as a high-resolution CT image[38], bringing great difficulty to the application of AI in surgery. To further verify the AI’s anatomic identifying capability, we compared the performance among SurgSmart, trainees, and seniors. Unexpectedly, the model’s overall IoU and correct identification rate are significantly higher than those of seniors and trainees on still images level. Although severe inflammation will seriously affect the anatomic identification for surgeons regardless of experience, it has no impact on the performance of SurgSmart. Furthermore, SurgSmart can correctly identify anatomic sites at earlier surgical phases than most surgeons. These results suggest that AI does well in identifying objects or scenes based on some characteristics hidden in still images, which are difficult to be detected or noticed by humans. Conversely, humans need more relational and logical information obtained through visual inspection, sequenced information, exploring by instruments, and the sense of touch, to identify complex objects. Hence, the omission of the latter three approaches during the experiment probably led to the low effective anatomic identification rate. Noticeably, the effective identification rate of the key region, such as the common bile duct in the senior group, approached over 90% when considering sequenced information. Therefore, the dependency of AI and humans on various information is quite different, leading to the potential necessity of intelligent guidance, especially for novice surgeons.
Interestingly, the shape of IoU curves in Figure 3 is very similar among the three groups, indicating the anatomic region that is complex for surgeons, as in the cystic artery, also impacts the recognition results of AI. Despite the difference between AI and trainees/seniors in terms of the depth of understanding of still images, some similarities might exist in their learning process. Besides, the performance of SurgSmart in images with severe inflammation tends to be better than those without severe inflammation, which is similar to the result of a recent CVS evaluation study performed by Korndorffer et al.[21] Therefore, utilizing the learning mechanism in humans may further improve the capability of AI in the future.
There are several limitations to the study. First, all surgical videos and images used for algorithm training came from limited institutes with restricted external access due to the hospital regulations. The current system, SurgSmart, may not be fully generalizable to other hospitals. To solve this problem, we have launched a multi-center project named “LC10000”, which will collect over 10,000 videos of LC in the next few years prospectively to expand the diversity of the dataset. Second, the comparison between the AI and surgeons only consisted of two cases, which consisted of 28 questions of still images required to select key areas of anatomic regions and might not directly translate to superior detection of anatomy during “live” surgery. Nonetheless, some participants felt overwhelmed and lassitude, which may also occur in the later stage of operations while facing a relentless set of tests, leading to poor results, especially near the end of the test. Thus, the distortion of results will generate if we expand the number of cases to incessantly evaluate the anatomic detection ability of surgeons, who would relentlessly answer many questions. Additionally, due to the low fault-tolerance rate of the surgical process, which means small mistakes may bring disastrous consequences to patients, it is difficult to achieve this by automated anatomy recognition only. Hence, combining various approaches, such as CVS evaluation, dangerous zone indication, and anatomic structure identification, would be helpful to enrich the AI system in the field of surgery.
In conclusion, SurgSmart can detect and localize key anatomic regions of LC accurately. In a series of still images, its anatomic locating ability is significantly more precise than those of surgeons. Although there is still a long way to go before SurgSmart can be utilized clinically, with the expansion of datasets and developments in the algorithm, we believe that a well-trained AI system will be born soon, translating the advantages of AI into benefits for patients and surgeons.
DECLARATIONS
Acknowledgements
We deeply grateful to the contribution of all the authors, and to the technical supports of Yuxian Wang, Zunyu Liu, and Shaonan Wu.
Authors’ Contributions
Study conception and design: Liu R, An J, Wang Z, Jiang J, Chen Z, Li H, Peng B, Wang X
Acquisition of data: An J, Guan J, Liu J, Wang Z, Chen Z, Li H, Wang X
Analysis and interpretation of data: Liu R, Guan J, Liu J, Jiang J, Chen Z, Li H
Drafting of manuscript: Liu R, Wang Z, Guan J, Liu J, Wang X
Critical revision: An J, Jiang J, Peng B, Wang X
Availability of data and materials
Due to the administrational issues of West China Hospital and its allies, the relevant data is temporarily not available to the public.
Financial support and sponsorship
This work is supported by key project of Health Commission of Sichuan Province, China (20ZD003).
Conflicts of interest
All authors declared that there is no conflict of interest.
Ethical approval and consent to participate
This study was approved by the Ethics Committee Board of West China HoWest China Hospital, Sichuan University, China. All procedures performed in studies involving human participants had been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
Supplementary Materials
REFERENCES
1. Soper NJ, Stockmann PT, Dunnegan DL, Ashley SW. Laparoscopic cholecystectomy. The new “gold standard”? Arch Surg 1992;127:917-21; discussion 921.
2. Schwaitzberg SD, Scott DJ, Jones DB, et al. Threefold increased bile duct injury rate is associated with less surgeon experience in an insurance claims database: more rigorous training in biliary surgery may be needed. Surg Endosc 2014;28:3068-73.
3. Törnqvist B, Strömberg C, Akre O, Enochsson L, Nilsson M. Selective intraoperative cholangiography and risk of bile duct injury during cholecystectomy. Br J Surg 2015;102:952-8.
4. Barrett M, Asbun HJ, Chien HL, Brunt LM, Telem DA. Bile duct injury and morbidity following cholecystectomy: a need for improvement. Surg Endosc 2018;32:1683-8.
5. Brunt LM, Deziel DJ, Telem DA, et al. the Prevention of Bile Duct Injury Consensus Work Group. Safe cholecystectomy multi-society practice guideline and state of the art consensus conference on prevention of bile duct injury during cholecystectomy. Ann Surg 2020;272:3-23.
6. Lilley EJ, Scott JW, Jiang W, et al. Intraoperative cholangiography during cholecystectomy among hospitalized medicare beneficiaries with non-neoplastic biliary disease. Am J Surg 2017;214:682-6.
7. Törnqvist B, Strömberg C, Persson G, Nilsson M. Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population based cohort study. BMJ 2012;345:e6457.
8. Fong ZV, Pitt HA, Strasberg SM, et al. California Cholecystectomy Group. Diminished survival in patients with bile leak and ductal injury: management strategy and outcomes. J Am Coll Surg 2018;226:568-576.e1.
9. Iwashita Y, Hibi T, Ohyama T, et al. Delphi consensus on bile duct injuries during laparoscopic cholecystectomy: an evolutionary cul-de-sac or the birth pangs of a new technical framework? J Hepatobiliary Pancreat Sci 2017;24:591-602.
10. Esteva A, Robicquet A, Ramsundar B, et al. A guide to deep learning in healthcare. Nat Med 2019;25:24-9.
12. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017;542:115-8.
13. Cicero M, Bilbily A, Colak E, et al. Training and validating a deep convolutional neural network for computer-aided detection and classification of abnormalities on frontal chest radiographs. Invest Radiol 2017;52:281-7.
14. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016;316:2402-10.
15. Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst 2019;111:916-22.
16. Xie Y, Zhang J, Xia Y. Semi-supervised adversarial model for benign-malignant lung nodule classification on chest CT. Med Image Anal 2019;57:237-48.
17. Grenda TR, Pradarelli JC, Dimick JB. Using surgical video to improve technique and skill. Ann Surg 2016;264:32-3.
18. Tan Mingxing, Le Quoc V. EfficientNet: rethinking model scaling for convolutional neural networks. 2019. Available from: http://proceedings.mlr.press/v97/tan19a/tan19a.pdf [Last accessed on 22 Apr 2022]
19. Anteby R, Horesh N, Soffer S, et al. Deep learning visual analysis in laparoscopic surgery: a systematic review and diagnostic test accuracy meta-analysis. Surg Endosc 2021;35:1521-33.
20. Yamazaki Y, Kanaji S, Matsuda T, et al. Automated surgical instrument detection from laparoscopic gastrectomy video images using an open source convolutional neural network platform. J Am Coll Surg 2020;230:725-732.e1.
21. Korndorffer JR Jr, Hawn MT, Spain DA, et al. Situating artificial intelligence in surgery: a focus on disease severity. Ann Surg 2020;272:523-8.
22. Twinanda AP, Yengera G, Mutter D, Marescaux J, Padoy N. RSDNet: Learning to predict remaining surgery duration from laparoscopic videos without manual annotations. IEEE Trans Med Imaging 2019;38:1069-78.
23. Abdelrahman T, Long J, Egan R, Lewis WG. Operative experience vs. competence: a curriculum concordance and learning curve analysis. J Surg Educ 2016;73:694-8.
24. Marchi D, Esposito MG, Gentile IG, Gilio F. Laparoscopic cholecystectomy: training, learning curve, and definition of expert. in: Agresta F, Campanile FC, Vettoretto N, editors. Laparoscopic cholecystectomy. Cham: Springer International Publishing; 2014. pp. 141-7.
25. Maier-Hein L, Wagner M, Ross T, et al. Heidelberg colorectal data set for surgical data science in the sensor operating room. Sci Data 2021;8:101.
26. Liu C, Guo Y, Li S, Chang F. ACF based region proposal extraction for YOLOv3 network towards high-performance cyclist detection in high resolution images. Sensors (Basel) 2019;19:E2671.
27. Lin TY, Maire M, Belongie S, et al. , editors. Microsoft COCO: common objects in context 2014; Cham: Springer International Publishing.
28. Madni TD, Leshikar DE, Minshall CT, et al. The Parkland grading scale for cholecystitis. Am J Surg 2018;215:625-30.
29. Movafegh F, Abbaszadeh A, Rassouli M, Lotfi MS, Nasiri M, Mokhlesi S. Development and validation of the Iranian version of the patient privacy and confidentiality scale. Indian J Med Ethics 2021;VI:1-13.
30. Kristen J. , Yoon-Soo P., Emil P. Are your assessment scores and feedback reliable? A statistical review for the surgical educator 2021. Available from: https://www.facs.org/education/division-of-education/publications/rise/articles/assessment-scores [Last accessed on 22 Apr 2022].
31. Barisoni L, Lafata KJ, Hewitt SM, Madabhushi A, Balis UGJ. Digital pathology and computational image analysis in nephropathology. Nat Rev Nephrol 2020;16:669-85.
32. Ruffle JK, Farmer AD, Aziz Q. Artificial intelligence-assisted gastroenterology- promises and pitfalls. Am J Gastroenterol 2019;114:422-8.
33. Madani A, Namazi B, Altieri MS, et al. Artificial intelligence for intraoperative guidance: using semantic segmentation to identify surgical anatomy during laparoscopic cholecystectomy. Ann Surg 2020; doi: 10.1097/SLA.0000000000004594.
34. Sato M, Koizumi M, Nakabayashi M, et al. Computer vision for total laparoscopic hysterectomy. Asian J Endosc Surg 2019;12:294-300.
35. Madad Zadeh S, Francois T, Calvet L, et al. SurgAI: deep learning for computerized laparoscopic image understanding in gynaecology. Surg Endosc 2020;34:5377-83.
36. Tokuyasu T, Iwashita Y, Matsunobu Y, et al. Development of an artificial intelligence system using deep learning to indicate anatomical landmarks during laparoscopic cholecystectomy. Surg Endosc 2021;35:1651-8.
37. Mascagni P, Vardazaryan A, Alapatt D, et al. Artificial intelligence for surgical safety: automatic assessment of the critical view of safety in laparoscopic cholecystectomy using deep learning. Ann Surg 2020; doi: 10.1097/SLA.0000000000004351.
Cite This Article
How to Cite
Liu, R.; An J.; Wang Z.; Guan J.; Liu J.; Jiang J.; Chen Z.; Li H.; Peng B.; Wang X. Artificial intelligence in laparoscopic cholecystectomy: does computer vision outperform human vision?. Art. Int. Surg. 2022, 2, 80-92. http://dx.doi.org/10.20517/ais.2022.04
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.